In recent decades, orthopedics has become one of the most dynamic medical specialties, a fact confirmed by the huge increase in the number of surgical interventions, as well as their complexity. Changes in lifestyle, which has become much more active and exposed to different types of accidents, the increase in life expectancy and, last but not least, significant technological advances are responsible for this advance. This fact leads to special situations, more and more frequent, characterized by different amounts of bone loss, i.e., situations that require the use of bone grafts or substitutes, depending on different circumstances. The used bone grafts can be natural or synthetic, each one with their advantages and disadvantages and special indications as well.
Bone healing is a complex process, conditioned by the interaction between physical factors and the biological response. A defining element of the unique bone healing process is that it is not achieved by creating a fibrous scar but by a process of tissue regeneration. Bone healing is of two kinds: direct and indirect.
Direct bone healing can occur only when anatomical reduction of the bone fragments and their rigid fixation is achieved, which means a minimal interfragmentary movement. Bone healing in this case is conducted in a special way, i.e., by contact or gap healing.
Indirect healing is most common in the natural evolution of fractures; it does not require anatomical reduction of the fracture or special stability of the fixation; moreover, the healing speed through this mechanism is improved by micromovements and especially by direct axial loading.
2. Bone Grafts and Substitutes for Bone Repair
2.1. Natural Bone Grafts and Substitute Materials
Materials of natural origin are defined as those materials that have been derived from a living source without modification. These materials can be divided into four categories: autologous materials, from the same individual—autografts; homologous materials, from another individual of the same species—allografts; heterologous materials from another species—xerographs and phytogenic materials [
13].
2.1.1. Autografts
Autografts are considered the “gold standard” among the different materials for bone augmentation [
14,
15]. This status is due to their osteogenic character, which keeps bone cell structures from the harvesting site alive [
16], but also to their osteoinductive properties which favor the differentiation of mesenchymal stem cells in osteoblasts due to their growth factors content [
17,
18]. Having an identical origin with the affected tissue means that the possibility of an unwanted immune rejection reaction is eliminated, achieving a success rate of over 95% [
19,
20].
The most frequent harvesting site for autografts is the iliac crest; from this region, both cortical and spongious graft can be harvested, depending on the necessities. On the other hand, the obtained bone can be arranged to adapt as best as possible to the receiving site. The other sites are distal femur (especially for a spongious graft), the peroneal shaft for structural graft, ribs and distal radius. In dentistry, autologous augmentation materials are usually obtained from the same individual, such as the mandibular symphysis, mandibular branch and external oblique ridge, but also from other donor places such as the iliac crest or distal area of the ulna due to good cortical and spongious bone resources [
21]. Autograft bone harvested from the mandibular branch presents low risks compared to other areas of the oral cavity. It must be taken into account that this harvest may endanger the inferior alveolar nerve. Tissue harvesting from the mandibular branch is suitable to use when the area of the receptor is less than 4 mm thick and extends to at most four teeth [
22].
Even though there are also other augmentation materials commonly used to manage bone defects, block autologous grafts are still commonly used in more complex oral augmentation procedures, as very few other augmentation materials can produce a volume of new bone tissue similar to those obtained after the use of autografts. This is due to the fact that these autogenous bone blocks improve the repaired bone structure both in terms of volume and quality, favoring the use of implants with a larger diameter that facilitate the proper distribution of forces [
23,
24,
25].
Moreover, in orthopedic treatments, autografts are considered the “gold standard”. Cancellous autografts are the most frequently used as they bring to the receiving site mesenchymal stem cells which provide the capacity to stimulate the formation of new tissue in the affected area [
26]. Cortical autografts offer an integral and rigid structure with special mechanical properties With a reamer–irrigator–aspirator system, the autologous material can be obtained directly from an intramedullary canal of long bones [
27,
28].
A series of disadvantages, such as the morbidity of the harvesting place and the reduced volume of tissue that can be obtained, limit the autografts used both in orthopedics and dentistry [
29]. The second surgical trauma frequently has an increased morbidity, sometimes even affecting the patient’s general condition, when it involves large tissue structures to be collected. Reduced bone supply is mentioned when harvesting areas are selected from the oral cavity, which also frequently correlates with local problems related to healing after the surgical act [
30,
31].
2.1.2. Allografts
Allografts are natural materials that come from an individual of the same species and can be obtained from a compatible living donor or from cadaveric bone sources. Allograft materials can be prepared in three main forms—fresh, frozen or freeze-dried [
32]. Fresh and frozen homologous materials have superior osteoinductive properties but are rarely used today due to the increased risk of a host immune response, limited vitality and increased risk of disease transmission [
33].
Allografts are commonly used in the United States, being preferred by orthopedic surgeons, and there are currently four bone tissue banks that deal with the procurement and processing of osteochondral tissue [
34]. The disadvantages of using allografts are generally reduced by lyophilization but also by other methods of tissue processing, such as mechanical debridement, ultrasonic washing and especially sterilization using gamma radiation [
35,
36].
In Europe, increased regulatory restrictions on the use of materials from other people have led to a shift in the frequency of use from these materials to synthetic augmentation materials [
37].
Allografts have good histocompatibility and are available in various forms, from whole bone segments, cortical–spongious segments and cortical pieces, to small pieces in the form of bone chips, powder and demineralized bone matrices. They can be produced in custom forms to meet the requirements of the receiving sites [
38].
Homologous bone blocks from bone banks have been commonly used to rehabilitate bone supply in cases of severe atrophy of the alveolar process; they allow a sufficient volume of bone to be obtained for implant placement. Although their use compared to autologous materials avoids the existence of a second operating field, the integration time to be used in implant surgery exceeds 12 months [
39].
The use of fragmented bone in the form of small pieces of spongy or cortical bone is indicated with encouraging results due to the increased osteoconductive potential, especially in the case of larger defects in the posterior maxillary area that require performing operations to lift the sinus membrane. These small fragments of spongy and cortical bone are usually used in a mixture with each other or with other categories of augmentation materials due to the increased risk of resorption of the spongy bone [
40]. Demineralized bone matrix (DBM) is a decalcified product for which obtaining an acidic solution is used to remove mineral components, leaving behind collagen, other proteins, bone morphogenic proteins (BMP), variable percentages of calcium phosphate and a small percentage of cell debris.
After decalcification, BMPs are released from the surrounding mineral structure and can fully exercise their osteoinductive potential, while the remaining collagen proteins in the matrix can provide a 3D configuration for the growth of host tissue capillaries, perivascular tissue and osteoprogenitor cells. In the meantime, the original cells and any bacteria in the allogeneic bone are removed, which could reduce the risk of immune rejection and infection [
41,
42].
The release of BMP-type osteoinductive growth factors have cause demineralized bone tissue allografts to be considered a gold standard in periodontal regeneration surgical techniques [
43,
44].
DBM-based augmentation materials have been used successfully both to improve the bone substrate prior to dental implant insertion [
45] and for peri-implant repair surgery [
46]; however, they are especially used as a supportive material for a number of biologically active substances [
47]. Moreover, the increasing use of DBM in dental applications is related to its use as a transport vehicle for a number of added excipients, such as glycerol, starch, hyaluronic acid or saline, which allows for good maneuverability and improved adaptability [
48]. Moreover, the use of DBM in the form of putties to preserve the height and thickness of the alveolar processes immediately after tooth extraction provided good clinical results both in terms of accessibility of application and obtaining a bone substrate favorable to implantation only 6 months after extraction [
49].
2.1.3. Xenografts
Xenografts are materials that are derived from a genetically different species from the host species.
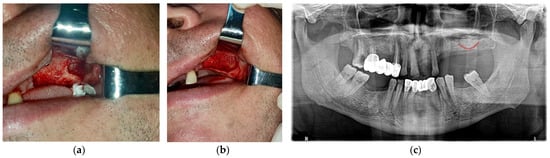
In dentistry, the most common source of xenograft material is deproteinized bovine bone tissue that is commercially available as Bio-Oss. Bovine bone is treated to produce a hydroxyapatite-based porous material that contains only the inorganic component of bovine bone tissue. The resulting porous structure closely resembles that of human bone and can provide good mechanical support and stimulate the healing process by osteoconduction. This porous structure facilitates the development of new blood vessels through angiogenesis, which underlies the formation of new bone tissue [
50]. Bone xenografts of bovine origin have been used extensively in procedures for lifting the floor of the maxillary sinus and obtaining implant support due to their superior stability and low immunogenicity (
Figure 1) [
51,
52].
Figure 1. Clinical aspects of the lateral bone window created in a direct sinus lift surgical intervention before (a) and after augmentation (b) with particles from a xenograft material, followed by the post-op radiological examination (c) (Courtesy of Dr. Salan Alex, University of Medicine and Pharmacy of Craiova, Romania).
Statistical studies have suggested that the effectiveness of Bio-Oss in stimulating the formation of new bone tissue is similar if not superior to autografts [
53,
54]. It was also found that the volume and quality of the newly obtained bone tissue allow for the foreseeable simultaneous placement of the implants, thus performing procedures for augmentation of the maxillary sinus in one stage [
55]. Clinically, Bio-Oss has been shown to be a valuable bone replacement material, providing good-quality newly formed bone structures and promising rates of long-term survival of inserted dental implants [
56]. Of course, there are other commercial products, such as OsteoGraf, Cerabone [
57] or Lumina-Porus [
58], which have very similar structures and biochemical properties favorable to human bone and which can act as an effective osteoconductive graft [
59].
Another xenograft material with promising results in the recent studies is chitosan, which is a polymer derived from crustacean exoskeletons capable of stimulating bone regeneration by providing a structural skeleton that supports osteoblastic activity, mineralization of bone matrix and induction of mesenchymal cell differentiation into osteoblasts [
60]. Due to its poor mechanical properties, chitosan is usually combined with other materials in order to obtain the desired properties. However, its structural versatility and hydrophilic surface make this material a viable alternative to autografts and allografts [
61].
2.1.4. Phytogenic Material (Plant, Coral, Marine Algae)
Phytogenic materials are bone augmentation materials from plant sources. A number of in vitro studies have suggested that plant-derived substances may induce osteogenic differentiation of stem cells and also have angiogenic potential. Moreover, in the field of tissue engineering, plant-derived compounds or plant extracts can be easily incorporated as biomaterials. However, the lack of predictive use, clinical efficacy and quality control are currently the major impediments to their widespread use [
62].
Corals, due to their chemical and structural characteristics similar to those of human spongious bone, have a high potential for use as a bone augmentation material, but the clinical data presented so far are ambiguous, with both positive and negative results reported. They have porous structures of different sizes, good compressive strength, low immunogenicity and good bonding with bone tissue, but have relatively low tensile strength, brittleness and a pattern of resorption that does not seem appropriate [
63].
The product of Frios AlgiPore is a seaweed hydroxyapatite that has been used clinically as a bone augmentation material since 1988, considered a favorable bone substitute material due to its excellent biocompatibility, low immunogenicity, biodegradability and bone binding capacity, but which was used more like a space maintainer after tooth extraction to maintain bone volume and avoid deformation of the edentulous ridge [
64].
2.1.5. Bone Graft Material Derived from Extracted Tooth Used in Dentistry
Bones, dentin and enamel have a similar composition to hydroxyapatite in the inorganic component as well as type 1 collagen and other proteins in the organic component but with different percentages [
65,
66]. The potential osteoinduction characteristics of the demineralized dentin matrix have been demonstrated in several studies, as well as the presence of bone-morphogenetic proteins in the human dentin matrix after a demineralization process [
67,
68].
In 2017, Rijal theorized how the process of dentin demineralization of autologous extracted teeth allows better bone augmentation through the increased availability of bone morphogenetic proteins [
69]. Other studies have confirmed the efficacy of a partially demineralized autologous dentin matrix prepared in real time for human bone regeneration clinical procedures [
70,
71].
Since bone and dentin are mineralized tissues with an almost similar chemical composition, and the morphogenetic proteins in dentin and bone have a major stimulating effect with osteoinductive properties, the regenerative properties of autogenous demineralized dentin matrix (DDM) have been highlighted in several studies. It was found that the dentinal collagen matrix, similar to the bone matrix, can also induce bone formation. They are currently in development, and there have already been a few clinical uses of such demineralized dentin (DDM) matrices, produced from the patient’s extracted teeth, to repair alveolar bone defects. The materials obtained are processed and then applied in the form of powders, bone blocks or moldable pastes, and in the future, they could be an option for use as a vehicle for growth factors and stem cells [
72,
73].
2.2. Synthetic Bone Substitute Materials
2.2.1. Calcium Phosphate Ceramics (CaP Ceramics)
Hydroxyapatite (HA) is the most widely used ceramic material for human bone augmentation because it has a chemical composition and a crystalline structure similar to that of bone. Its bioactivity is related to osteoconductive properties, which allow the apposition and migration of osteoblasts to the surface of the material [
74,
75,
76]. HA, alone or in combination with an auto-/allo-/xenograft, has been used with adequate clinical success rates in dentistry and orthopedics to support bone regeneration [
77]. However, the quality and quantity of newly formed bone following augmentation with synthetic HA only was often considered insufficient. This is why recent research has focused on the production of HA particles with nanometric dimensions, which improves the biomechanical properties and better mimics the composition of natural bone [
78,
79]. The nanostructure allows a higher surface-to-volume ratio, favoring the adhesion, proliferation and differentiation of osteogenic progenitor cells [
80,
81,
82].
Tricalcium phosphate (TCP) has two crystallographic forms: α-TCP and β -TCP. β-TCP is a material that has been widely used as a bone replacement material for many years. It has a faster biodegradation and absorption compared to HA due to its low level of Ca/P ratio, but it also has many desirable properties, such as ease of handling, radiopacity that allows monitoring of healing, good osteoconductivity due to macroporosity that promotes fibrovascular growth and osteogenic cell adhesion, good resorbability compared to bovine bone grafts, low immunogenicity and no risk of disease transmission [
83]. While the interconnected porous structure of β-TCP allows for improved vascularity, it also causes poor mechanical strength which makes it suitable as a bone substitute only with other materials, especially hydroxyapatite [
84].
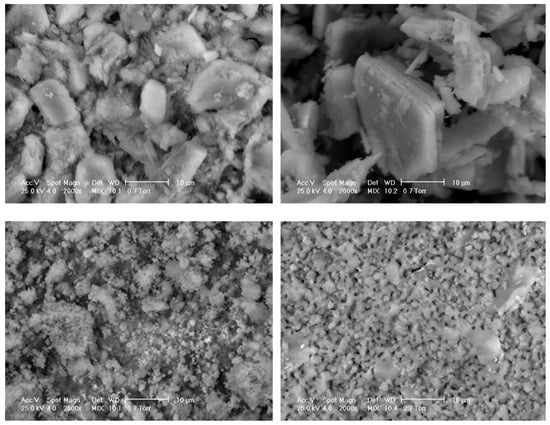
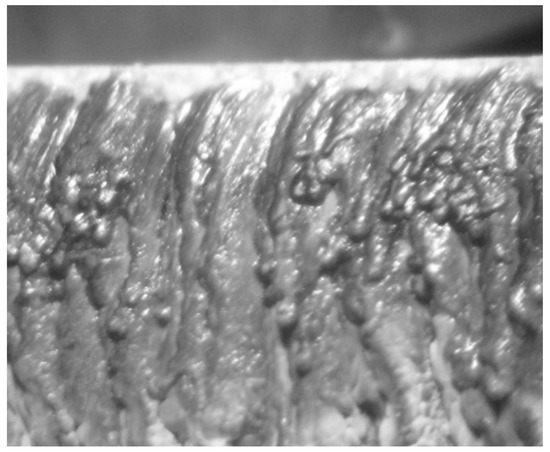
That is why β-TCP and HA are frequently used in combination today, developing biphasic commercial products (Figure 2).
Figure 2. Scanning electron microscopy images of different commercial bone substitutes based on calcium phosphate ceramics (Courtesy of Prof. I.V. Antoniac, University Politehnica of Bucharest, Faculty of Materials Science and Engineering).
Therefore, faster and higher bone regeneration rates were obtained compared to using only HA, but also better mechanical properties than β-TCP used alone. In addition, the resorption and osteoconductivity of these biphasic calcium phosphate ceramics can be controlled by changing the HA/β-TCP ratio (
Figure 3 and
Figure 4) [
85].
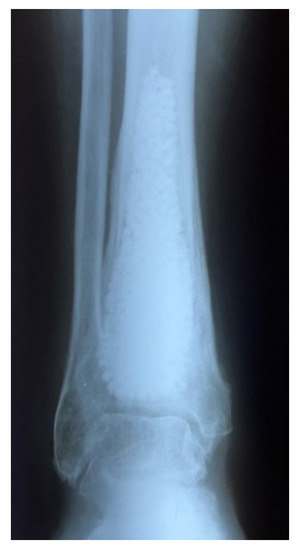
Figure 3. Filling the remaining cavity after intralesional curettage of a tumor with granules of synthetic biphasic ceramic (hydroxyapatite HA and beta tricalcium phosphate β-TCP).
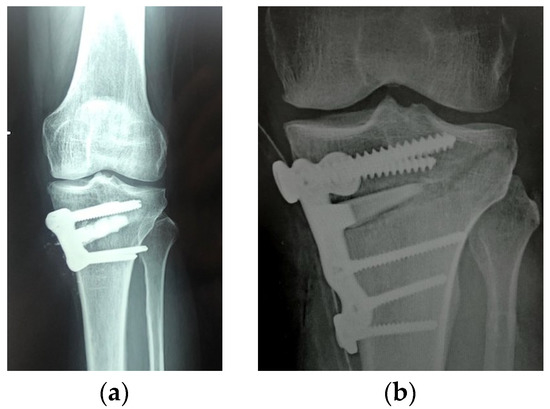
Figure 4. Synthetic biphasic ceramic (hydroxyapatite HA and beta tricalcium phosphate β-TCP) granules (a) vs. wedge (b) inserted into the high tibial osteotomy gap. The structural graft provides greater stability and strength to the construct.
2.2.2. Calcium Phosphate Cements (CPC)
Calcium phosphate cements are two- or three-component systems that typically contain materials such as TCP and HA. The mixing of the components results in a paste that hardens in situ to form HA nanocrystals at room temperature. The main advantages of these cements include their ability to form a pasty consistency instead of the defect, their ability to replicate the structure and composition of the bone in a repeatable manner, their high biocompatibility, their availability in different forms, for different types of bone defects and properties, and their osteoconductivity. However, they lack a macroporous structure which limits the rate of cell adhesion, fluid exchange and restoration capacity. In addition, the risk of incomplete setting may lead to a local inflammatory reaction. Recent research proposes the development of 3D-printed structures prefabricated from these cements and their improvement through various mechanisms including the addition of viscous binders such as chitosan, gelatin and hyaluronic acid, optimizing the size, distribution and particle shape or optimizing the setting reaction [
86,
87].
2.2.3. Composite Bone Substitute Materials
Composites are one of the advanced materials used in various kinds of bioapplications. By definition, they represent a mixture between one continuous component, named “matrix”, and one or more components (continuous or discontinuous), named “reinforcement”(s). The symbol of a composite is generally represented by the formula: matrix material/reinforcement material/reinforcing content (wt.%). For instance, HA/HDPE/27 means a composite material made of hydroxyapatite as a matrix, which is reinforced by high-density polyethylene of 27% wt.
The choice to select a biocomposite or a biomaterial for a specific application, i.e., bone substitute materials, is made according to functional, technological and (not the least) economic reasons. The properties provided by the conventional biomaterials (metallic or ceramic or polymers) are generated during their processing (by physical–chemical reactions between the chemical elements) but especially by post-processing operations (such as mechanical, thermal, biofunctional, etc.) involving extra-time and energy consumption costs, respectively.
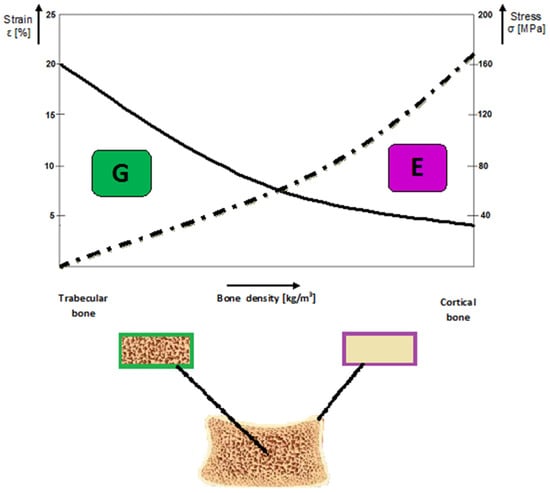
Graphically, the recommendations to use the composites for BS may be expressed as functions of the bone density and the driven mechanical properties governing the alloplastic composite selection, respectively (Figure 5).
Figure 5. Graphical representation on the general prediction concerning the relationship between the structural features of the bone tissues and the governing mechanical properties for a specific grafting application (Courtesy of Prof. Oana Gingu, University of Craiova, Faculty of Mechanics).
On the other hand, in order to obtain a particular property for a bone substitute material, the matrix and reinforcements are optimally selected from different points of view (structural, functional, market availability, biocompatible and environmentally friendly) to fulfil the most efficient ratio between the expected performance and necessary processing costs. The most important feature in designing a biocomposite is to create a working interface between the matrix and reinforcements, i.e., one able to efficiently transfer the loads (mechanical, thermal, etc.) during its functioning without any components’ physical and chemical degradation.
As far as the bone substitutes are concerned, biocomposites provide a wide range of possibilities to significantly improve the properties of the above-mentioned conventional solutions (autologous grafts, allografts and xenografts). Different types of biocomposites are further presented—But also, their selection criteria for a specific bone substitute.
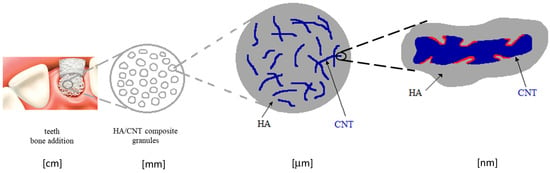
The mechanical properties of HA may be significantly improved by its reinforcing with carbon nanotubes (CNTs, 1–3 wt.%). Most of such applications concern dentistry applications [
88]. The obtained properties were superior vs. the pure HA, such as the flexural strength, 83 MPa (1.6 times higher); and the fracture toughness, 2.4 MPa m
1/2 (2 times higher), because of the special interface geometry between the matrix and the reinforcing element, schematically represented in
Figure 6. The higher is the CNT roughness, the more efficient is the interlocking effect towards the HA particles whose properties are provided by the processing technology.
Figure 6. Schematic representation of the micromechanical interlock model of the interface between the HA and CNTs, providing a physical bonding at the interface level (red curves).
The processing of such interface morphology was released by means of the double in situ CNTs synthesis within the HA matrix by chemical vapor deposition (CVD) using Fe catalysts [
89].
An alternative approach to reinforce the matrix of a biocomposite is to use precursors, leading to in situ synthesis of the reinforcements. Among the processing technologies for bone substitutes, powder metallurgy (PM) is one of the most versatile. The reasons lie in its flexibility to select the adequate materials for the matrix and the reinforcing precursor, the reinforcing content and the particle size of both components. Another important aspect is the selection of the sintering processes and parameters, providing the physical chemical conditions for the precursor’s reactions and leading to the optimal reinforcing effect of the matrix.
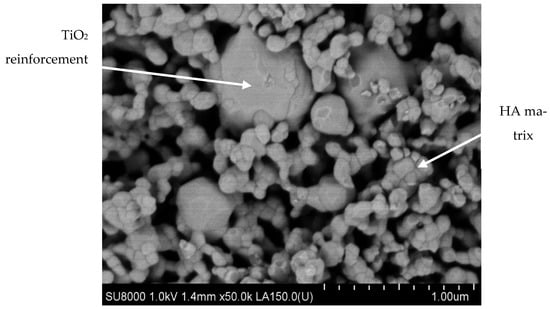
Recent research regarding advanced materials for alloplastic bone substitutes highlighted the PM biocomposites based on submicronic HA particles reinforced by titanium hydride micrometric powders (100–150 µm; 25% wt.) as the precursor. The TiH
2 dehydrogenation reaction [
89] occurring during the two-step sintering (TSS) treatment leads to the biocompatible TiO
2-rutile allotropic phase synthesis [
90,
91], which acts as a reinforcement for the HA/TiO
2 sintered biocomposite (
Figure 7).
Figure 7. SEM micrograph of HA/TiO2 biocomposite processed by TSS technology (Courtesy of Prof. Oana Gingu, University of Craiova, Faculty of Mechanics).
On the other hand, the hydrogen releasing during dehydrogenation reaction determines a specific porosity; thus, alloplastic bone substitutes or trabecular bone tissues may be designed using this technological approaching corroborated with the reinforcing content. Moreover, the PM biocomposites’ porosity could be increased (30–60%) using different foaming agents such as calcium carbonate (CaCO
3) and ammonium hydrogen carbonate (NH
4HCO
3) in the initial powder mixture. The TSS technology allows for the monitoring of the foaming reactions; thus, each foaming agent contributes to creating the specific morphology of the pores [
92].
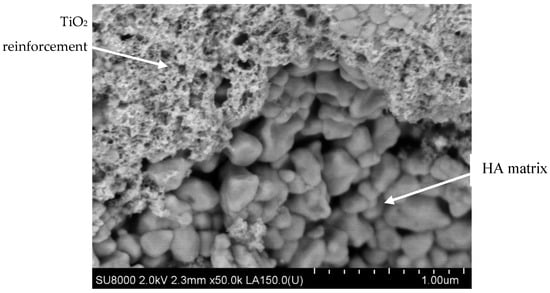
For the cortical bone tissue, BS applications could be manufactured by PM biocomposites obtained through spark plasma sintering (SPS) technology. The same dehydrogenation reaction mentioned above for HA/TiO
2 composite is restricted by the compaction developing simultaneously with the sintering treatment, so the porosity is much lower (5–12%) than in the case of the same biocomposite made via TSS (
Figure 8) [
93].
Figure 8. SEM micrograph of HA/TiO2 biocomposite processed by SPS technology (Courtesy of Prof. Oana Gingu, University of Craiova, Faculty of Mechanics).
For some clinical cases, the necessity for geometrical matching between the alloplastic bone substitutes product and the bone defect arises. Recent research proved that the biocomposite type HA/TiO
2 manufactured via TSS technology presents high potential to be processed by laser micromachining in order to fit the bone defect (
Figure 9). Using a pulsed Nd:YAG solid state laser with 1064 nm wave length, 4 mm laser beam diameter, pulse energy of 15 J max., average power of 1000 W and specific cutting regimes (pulse length, 0.02–20 ms; pulse frequency, 0.1–1000 Hz; and voltage, 240–320 V), the outer surface of the graft presents a variable roughness (Ra = 4.6–18.2 µm), assuring good physical interface with the adjacent natural bone without cracks/fractures in the bulk composite bone substitutes [
94].
Figure 9. Macroscopic aspect (×8 magnification) of HAP/TiO2 biocomposites after laser micromachining at 280 V, 60 Hz and 0.35 ms as pulse duration. The average obtained roughness Ra = 15.4 µm.
The bone substitutes’ biocompatibility is significantly increased using submicronic HA powder particles as ceramic matrix. According to Varut et al., different antibiotics (such as ciprofloxacin, gentamicin) may be adsorbed on the nanostructured HA/Ti biocomposites, and their releasing in a few days provides a significant osteointegration support under antibacterial protection.
Furthermore, these effects are more intense in the case of calcium fructoborate (CaFB) adsorption on the HA/TiO
2 PM biocomposites [
95,
96].
The nanostructured HA matrix, which is TiO
2-rutile reinforced by the TSS treatment, is responsible for other improved biocompatible performances. The cell viability of L929 fibroblast cells, assessed via quantitative (MTT assay) and qualitative (Giemsa staining) tests in connection with morphological analysis, demonstrated increased biocompatibility in comparison with HA-based ceramics. Moreover, the nanostructured HA proved to be stable, i.e., its decomposition to phosphates is avoided during the TSS treatment [
90]. Other in vitro tests using mesenchymal stem cell culture confirms an improved biocompatibility of HA/TiO
2 PM biocomposites [
97], and CaFB functionalization increases this property, i.e., the osteointegration process [
98] (
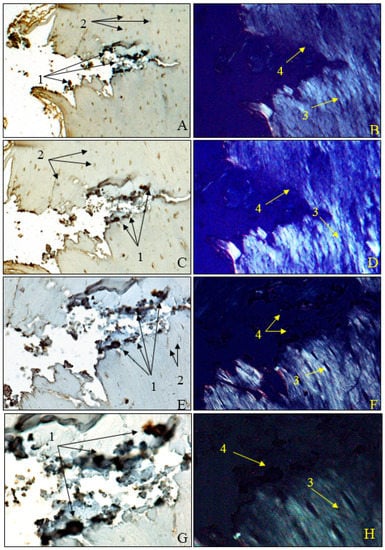
Figure 10).
Figure 10. Morphogenesis of implant integration into adjacent bone tissue; (A–H) tissue surrounding the HApTiCaFb implant in the right femur was immunostained for OPN. 1: adherent fragments of adjacent bone-bearing tissue; 2: osteocytes; 3: birefringent collagen fibers under polarized light examination; 4: implant incorporation zone without the birefringence phenomenon. Anti-OPN antibody immunostaining: (A,B) ×28; (C,D) ×140; (E,F) ×210; (G,H) ×280. HApTi: Hydroxyapatite-coated titanium; HApTiCaFb: Calcium fructoborate coating on a HApTi; OPN: Osteopontin.
Another category of composite materials with interesting properties in this field starts from the natural mammalian bone matrix formed from hydroxyapatite and collagen. This structure represents a matrix on which various other components, such as Ag, Sr and Zn, can be added. The addition of magnesium can also reduce the rate of bone substitutes resorption, an important aspect in a number of clinical situations [
99] (
Figure 11).
Figure 11. Experimental composite materials for bone substitutes type collagen–hydroxyapatite (first line) and collagen–hydroxyapatite–magnesium (second line): left row (up and bottom) macroscopic view, middle row (up and bottom) scanning electron microscopy image, right row (up and bottom) transmission electron microscopy image.
Synthetic materials with a polymer matrix to which hydroxyapatite powder can be added also demonstrate an interesting potential [
100].