1. Introduction
Baker’s yeast
S. cerevisiae was the first eukaryotic system used for heterologous expression. Yeast genetics and physiology are well characterized, and this yeast remains the most helpful tool to elucidate the functions of proteins of different origins [
14]. The convenience of this model system for studying, in particular, plant proteins is explained not only by the multiple simple and available routine methods of yeast manipulation but also by the fact that yeasts possess a eukaryotic system of posttranslational modifications of synthesised proteins. At present, there exists a large collection of
S. cerevisiae mutants that are used for functional studies of heterologously expressed proteins (
http://www.yeastgenome.org (accessed on 25 June 2023); [
9]). The cells of
S. cerevisiae successfully served as vehicles for the expression of many proteins of plant origin, including membrane proteins such as phosphate transporters AtPT1 и AtPT2 [
15], Ca
2+-ATPase of endoplasmic reticulum ECA1 [
16], nitrate transporter NRT1.1 [
17], peptide transporter of plasma membrane AtPTR1 [
18] (all of these proteins are from the model plant
A. thaliana), sulphate transporters LeST1-1 and LeST1-2 from tomato
Lycopersicon esculentum [
19] and Na
+-ATPase PpENA1 from moss
Physcomitrella patens [
20]. To identify stress-tolerance-related plant genes the functional screening of the genes in yeast can be applied. For example, for the halophyte
Ipomea pes-caprae, large-scale screening of genes involved in the salt stress response was performed via a full-length cDNA over-expressing gene hunting system (FOX hunting system, the gain-of-function system [
21]) with a functional screening of a cDNA library using a salt-sensitive yeast mutant strain [
22].
2. Vector Systems Used for Heterologous Expression in Cells of S. cerevisiae
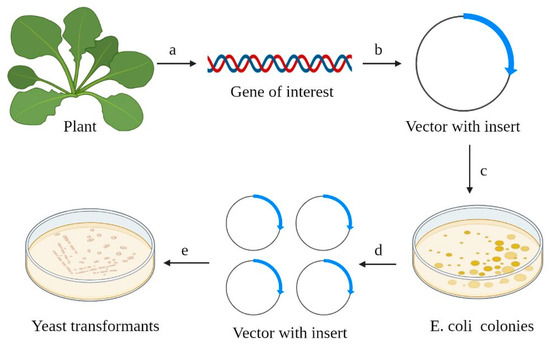
Figure 1 shows the scheme of an experiment on cloning the gene of interest from plants in yeast cells. All types of plasmids used for the design of constructions of heterologous expression in yeast cells are binary (or shuttle) vectors [
12,
23]. They contain the sequences derived from bacterial plasmids and the components of a yeast replication system, permitting them to replicate in both
E. coli cells and yeast cells. The ability to replicate in
E. coli cells ensures the simplicity of manipulations and the convenience to obtain large amounts of plasmids.
Figure 1. The key steps in the expression of heterologous protein in yeast cells. (a) The gene of interest (cDNA) is isolated from a plant sample; (b) the cDNA (blue arrow) is integrated into the host vector; (c,d) E. coli is routinely used for construct amplification; (e) yeast cells are transformed by the vector containing the cDNA of gene of interest. Selection of colonies (E. coli, yeast colonies) is carried out on selective media.
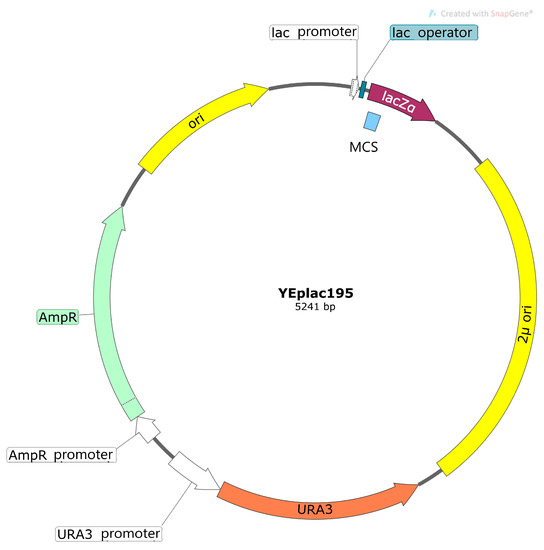
The bacterial segment of the plasmid has elements that are required for plasmid replication in E. coli cells: bacterial ori (replication initiation site) and the selective gene of antibiotic resistance (for example, resistance to ampicillin) (Figure 2). Multiple unique restriction sites located in the region of the plasmid named polylinker allow the insertion of a heterologous coding sequence. The yeast part of the plasmid, in addition to the elements necessary for replication in the yeast cell (ori, ARS) or integration into its genome, contains additional selective markers for the selection of yeast transformants on selective media. For example, genes of β-isopropylmalate dehydrogenase (LEU2) or orotidine-5′phosphate decarboxylase (URA3) are often used for this purpose. Yeast strains deficient in the LEU2 gene or URA3 gene and subsequently auxotrophic in the components of growth medium (leucine or uracil, correspondingly) are used for transformation. It allows the selection of yeast transformants in media without leucine or, correspondingly, uracil.
Figure 2. Scheme of yeast episome plasmid. MCS—multiple cloning site (polylinker); ori—bacterial replication site; AmpR—gene of resistance to ampicillin (selective markers for the selection of bacterial transformants); 2µ ori—ori site of yeast 2µ-plasmid; URA3—gene of orotidine-5′phosphate decarboxylase (selective markers for the selection of yeast transformants).
Based on the manner of replication in the yeast cells, the genetic constructions for the expression of heterologous proteins in
S. cerevisiae can be classified into four groups [
12]: YIp (yeast integrating plasmids), YRp (yeast replicative plasmids), YCp (yeast centromere plasmids) and YEp (Yeast Episome plasmids).
Integrative plasmids YIp do not contain yeast ori sites and are replicated when integrated into the yeast genome. They contain regions of homology with yeast DNA, and the integration into the genome is realised by homologous recombination. The vectors are stable, but typically the number of their copies in the genome is relatively low, which determines the low level of heterologous protein expression. This problem can be solved by integrating vectors into the region of tandem repeats of the rRNA gene. This integration allows for many copies of the cloned DNA sequences. In addition, the integration site can profoundly affect the levels of exogenous gene expression [
24]. Integrative vectors find their applications in metabolic engineering in yeast, which often require stable integration of heterologous genes [
25,
26].
The replicative plasmids YRp, in addition to the vector sequence of YIp type, contain the yeast chromosome replication initiation site ARS (autonomously replicating sequence). Plasmids of the YRp type can autonomously replicate in yeast cells without integration into the chromosomes. However, after yeast cell divisions, YRp plasmids are distributed unevenly in daughter cells, and the progeny cells may lose the plasmids.
The constructions of YEp-type plasmids are based on episomal vectors that differ from integrative plasmids by the presence of the ori site of the yeast 2μ-plasmid (Figure 2). Their replication is independent of chromosome DNA; they are present in cells with large copy numbers (30 copies or more). The plasmids distribute unevenly in the progeny cells, but they are supported consistently due to the large copy number. Plasmids of the YEp type are used for achieving high expression levels of heterologous proteins. In many cases, the amount of the expressed protein is the most important factor, and these high-copy vectors are routinely used for the overexpression of recombinant proteins.
The vectors of the YCp type contain a yeast centromere sequence (CEN) fused to ARS. The fused sequence ARS/CEN is responsible for the replication and segregation of the YCp-type vectors. The vectors, as YEp-type vectors, replicate independently of chromosome DNA; they are stable and evenly distributed between the daughter cells, but they are represented by a small number of copies.
To express a heterologous protein, the coding part of its gene is integrated between the promoter of the yeast gene with high expression and its terminator [
12]. One of the advantages of yeast systems is the presence of strong constitutive promoters. In
S. cerevisiae, these promoters control the expression of proteins such as plasma membrane H
+-ATPase (
PMA1), glyceraldehyde-3-phosphate hydrogenase (
GPD), phosphoglycerate kinase-1 (
PGK1), alcohol dehydrogenase-1 (
ADH1), and the transporter responsible for pleiotropic drug resistance (
PDR5) [
27]. Apart from the constitutive promoters, there are inducible promoters that can ensure the expression of heterologous proteins at a required time. This is especially important when expressing proteins that are toxic to cells. The set of inducible promoters includes promoters of galactose metabolism
GAL1-10 (induced by galactose),
PHO5 (induced by low concentrations of inorganic phosphate in an external medium) and
HSE elements (induced by an increase in the temperature to 37 °C). A description of yeast strains used in laboratory practice, expression vectors, selection markers and promoters for recombinant protein production in various yeast strains is reviewed in [
28].
3. Functional Complementation of Yeast Saccharomyces cerevisiae as a Method to Study Plant Potassium and Sodium Channels and Transporters
Most processes controlling ion homeostasis are essentially similar for cells of higher plants and
S. cerevisiae [
29]; therefore, the cells of
S. cerevisiae are suitable as a model system to study ion transporters of plant origin. Some of the most successful examples of exploiting
S. cerevisiae for heterologous expression of plant membrane proteins and their functional complementation are the studies of proteins that transport potassium and sodium.
There are several general experimental approaches to identify the genes of potassium and sodium transporters, determine the kinetic properties of the transporters and find their potential regulators employing
S. cerevisiae: functional complementation using yeast mutants, high-throughput protein–protein interaction assay, the reconstitution of functional transport systems and the identification of plant genes capable of conferring salt tolerance upon overexpression (reviewed in [
30]). One of the most widely used approaches to functionally characterise the transporters of K
+ and Na
+ is the functional complementation of yeast
S. cerevisiae mutants that are defective in their own genes for the corresponding transporters.
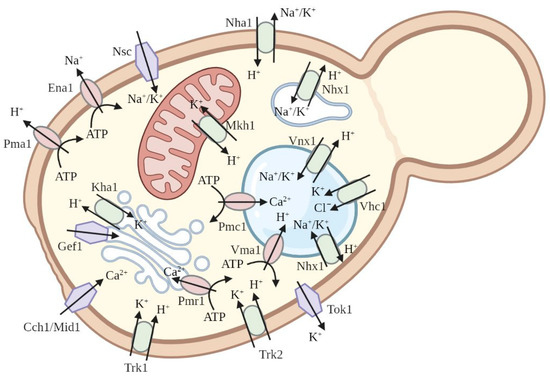
Baker’s yeast
S. cerevisiae possesses its own systems for potassium and sodium transport (
Figure 3). The uptake of potassium from the external medium by the cells of
S. cerevisiae depends mostly on two transporters in the yeast plasma membrane, Trk1 and Trk2 [
31,
32,
33]. These integral membrane proteins ensure high-affinity potassium transport at the expense of the electrochemical gradient of H
+ that is created by the yeast plasma membrane H
+-ATPase Pma1 [
34]. The yeast double mutant
trk1 trk2 is not able to grow in media with low concentrations of K
+ (below 1 mM), but the growth of the double mutant is restored to the rate of the wild-type yeast when millimolar concentrations of K
+ are added to the medium. Therefore, the double mutant
trk1 trk2 could be used for functional complementation when the heterologous expression of potassium transporters from other organisms in the mutant restores its growth under low K
+ concentrations in the medium.
Figure 3. The major known plasma membrane and intracellular membrane ion transporters in the yeast cell are mentioned in the text.
Under conditions of salt stress with high external Na
+ concentration, Na
+ enters the yeast cell via nonselective cation transporters in the plasma membrane (Nsc) and also via the potassium transporters Trk1 and Trk2 [
33,
35]. Then, two main systems function to expel Na
+ from the cytoplasm of yeast cells. The first system is the P-type Na
+-ATPase Ena1 [
36,
37], which is located in the plasma membrane. Ena1 actively pumps Na
+ out of the cytoplasm and hydrolyses ATP to provide energy for this process. The genomes of many yeasts possess three to five tandem copies of the
ENA gene;
ENA1 expression is induced upon exposure to salt and alkaline stresses [
38]. When the external pH of yeast cells is low, the other system of Na
+ export from the cytoplasm plays an important role, which is represented by the integral membrane protein Nha1 [
39,
40]. The Nha1 transporter functions as a cation/H
+ antiporter with presumably similar selectivities for Na
+ and K
+ (reviewed in [
41]). Strains lacking the
ENA cluster and
NHA1 gene are highly salt sensitive [
36,
42]. Such strains are used to characterize plant genes involved in cation extrusion and salt tolerance.
The cells of
S. cerevisiae are also equipped with additional transporters of potassium and sodium located at the vacuolar membrane. This group of transporters includes two proteins, Vnx1 and Vhc1. Protein Vnx1 is a cation/H
+ antiporter that transports potassium or sodium to the vacuole [
43]. Therefore, it participates in the detoxification of sodium via vacuolar compartmentalisation. The other protein of vacuolar membranes, Vhc1, is a K
+/Cl
− symporter that takes part in maintaining the intracellular concentration of potassium and the morphology of the vacuole [
44].
Other yeast cell compartments also have several ion transport proteins with selectivity for potassium and sodium. For example, the cation/H
+ antiporter Nhx1 with high affinity to Na
+ and K
+ is located in the tonoplast and endosomal membranes; K
+/H
+- antiporter Kha1 functions in the Golgi apparatus; protein Mkh1, which exchanges H
+ for K
+, was detected in mitochondria [
45].
The identification of plant genes involved in K
+ uptake (K
+ channels and transporters) is based mainly on complementation analysis of yeast mutants lacking
TRK1 and
TRK2 genes. The first plant potassium channels whose functions were proven by functional complementation of yeast
trk1 trk2 mutants (without their own high-affinity K
+-transporters) were the proteins KAT1 [
46] and AKT1 [
47] from
A. thaliana. Both proteins are inward-rectifying potassium channels belonging to the voltage-gated Shaker-type ion channel family; both are integral proteins of the plant cell plasma membrane, but they have different patterns of expression. KAT1 is expressed mainly in stomatal guard cells, where it takes part in the stomatal opening, whereas AKT1 is expressed in roots and is important for potassium uptake from soil [
48]. Under heterologous expression in
S. cerevisiae, each of the proteins was able to rescue the phenotype (restore the growth) of the double mutant
trk1 trk2 in a medium with low potassium [
46,
47]. Isoforms of KAT1 from other plants have also been identified (reviewed in [
30]). For example, using a
trk1 trk2 mutant, a KAT1 homologue from rice (
Oriza sativa) was characterized. The heterologous expression of OsKAT1 suppressed the K
+-transport defective phenotype of
trk1 trk2, suggesting the enhancement of K
+ uptake by OsKAT1 [
49].
Later, the approach with double mutant
trk1 trk2 was applied for the functional studies of other transporters, including plant high-affinity potassium transporters of the HKT family (high-affinity K
+ Transporter), which transport K
+ as well as Na
+ in plants [
50]. The phylogenetic and functional analyses of proteins belonging to the HKT family of transporters divide them into two groups, and the analyses added many details for their selectivity and functions [
51,
52]. Group I of HKT transporters includes transporters that mostly transport Na
+ via the plasma membrane; they are involved in the recirculation of the ion via vascular tissues, whereas the HKT transporters of group II function as Na
+-K
+-symporters or K
+-selective uniporters [
52,
53]. To date, the HKT transporters of group II have only been identified in monocotyledonous plants [
51]. Higher sodium selectivity for HKT from group I is linked to a conserved serine residue forming a motif of S-G-G-G in the first pore loop, whereas the ability to transport K
+ (and also Na
+, depending on concentrations) for group II HKT transporters is associated with a glycine residue at the position (G-G-G-G motif) [
52,
53]. HKT transporters are of special interest since several members of this family play important roles in the salinity tolerance of plants [
54,
55]. The
Arabidopsis AtHKT1 transporter has been shown to be a strong determinant of the salt tolerance of this species; it was demonstrated that AtHKT1 for Arabidopsis is located mainly in the phloem and also at the plasma membrane of xylem parenchyma cells [
56,
57]. Therefore, HKT’s role in the salinity tolerance of plants could be explained by the uptake of Na
+ from xylem vessels, recirculation of Na
+ via the phloem, and for glycophytes, prevention of accumulation of the toxic cation in photosynthetic tissues [
56,
57,
58].
The first functionally characterised HKT transporter was HKT1 from wheat (
Triticum aestivum; the protein TaHKT2;1 in modern classification); this protein was the first Na
+-coupled K
+-transporter described in higher plants [
50,
59]. HKT1 demonstrated properties of high-affinity Na
+-K
+-symporter when heterologously expressed in yeast
trk1 trk2 double mutant [
60]. For similar concentrations of potassium and sodium, HKT1 transports/symports one Na
+ with K
+; for higher (millimolar) concentrations of sodium, Na
+ ions can compete with K
+ and substitute K
+ in the cation binding sites of the transporter [
59,
60]. Therefore, at toxic (millimolar) levels of Na
+, HKT1 mediates low-affinity Na
+ uptake, whereas K
+ uptake via the transporter is blocked [
59]. Later, AtHKT1;1, the
Arabidopsis protein homologue of the wheat HKT1 (TaHKT2;1) was identified; when expressed in heterologous systems such as
S. cerevisiae (and
Xenopus laevis oocytes), AtHKT1;1 shows a strong preference for Na
+ selective transport [
61].
Recently, a novel HKT gene,
SeHKT1;2, was isolated and characterised from the halophyte
Salicornia europaea [
62]. The protein SeHKT1;2 belongs to subfamily I of HKT and shows high homology with other halophyte HKT proteins.
SeHKT1;2 expressed in the yeast
trk1 trk2 double mutant could not rescue the K
+ uptake-defective phenotype of this strain. However, functional characterisation of SeHKT1;2 in yeast strains with disrupted
ENA1-4 genes encoding the isoforms of yeast Na
+ exporting ATPase revealed that SeHKT1;2 contributes to facilitating Na
+ uptake in this Na
+-sensitive yeast strain, demonstrating that SeHKT1;2 selectively transports Na
+ rather than K
+.
In the
S. cerevisiae mutant
trk1 trk2, the low-affinity cation transporter (LCT1) from wheat was cloned and functionally characterised [
63].
LCT1 is expressed in low abundance in wheat roots and leaves. The expression of
LCT1 in
S. cerevisiae demonstrated that the transporter LCT1 mediated low-affinity uptake of Na
+ and Rb
+ and could function as a component of multiple low-affinity Na
+ and K
+ uptake pathways in wheat roots. Further experiments have shown that (1) LCT1 complemented a yeast disruption mutant in the
MID1 gene, a non-
LCT1-homologous yeast gene encoding a protein required for Ca
2+ influx and mating [
64], and (2) LCT1 mediates the high-affinity uptake of Ca
2+ and Cd
2+ into yeast cells [
65]. The authors concluded that LCT1 may contribute to the transport of the toxic Cd
2+ across plant membranes.
Apart from potassium channels and transporters, the functional complementation of yeast mutants led to the characterisation of Na
+-specific plant transport proteins. These include the tonoplast Na
+/H
+-antiporter AtNHX1 from the cells of
A. thaliana [
66]. The protein is homologous to the Nhx1 yeast transporter and complements the corresponding salt-sensitive
nhx1 mutant of
S. cerevisiae [
67,
68]. Later, a family of
AtNHX1-like genes of
A. thaliana (
AtNHX1-5), encoding for vacuolar-type Na
+/H
+ antiporters, was cloned and functionally characterised by their heterologous expression in yeast mutant
nhx1 [
69]. The expression of all the
AtNHX members of the family provided a recovery of the salt-sensitive yeast mutant, supporting their role in Na
+/H
+ exchange.
The functional complementation of the yeast
nhx1 mutant led to the characterisation of genes for NHX transporters from other plants, e.g., ThNHX1 protein from
Thellungiella halophila (renamed as
Eutrema salsugineum, a salt-tolerant relative of
A. thaliana) [
70], and TaNHX2 from wheat [
71]. Both proteins have significant sequence homology with the NHX sodium exchanger in
Arabidopsis. ThNHX1 presumably functions as a tonoplast Na
+/H
+ antiporter and plays an important role in the salt tolerance of
T. halophila [
70]. TaNHX2 encodes a K
+/H
+ antiporter of endomembranes; it is involved in cellular pH regulation and potassium nutrition under normal conditions [
71].
The examples described in this section are far from providing a comprehensive list of studies with plant K
+- and Na
+-transporters that use yeast mutants. For a more detailed description, readers can refer to the extensive review by Locascio et al. [
30], which is completely devoted to the subject.
4. Functional Complementation of S. cerevisiae Mutants to Study Other Plant Ion Channels
Heterologous expression in yeast mutants has been used to characterize the functions of some other plant channels. Examples of such studies include plant ion channels controlled by cyclic nucleotides (CNGCs, cyclic nucleotide-gated channels) [
72]. Most plant CNGCs belong to non-selective cation channels residing in the plasma membrane [
73]. They are formed by four subunits that are activated by cyclic nucleotide monophosphates, adenosine 3′,5′-cyclic monophosphate (cAMP) and guanosine 3′,5′-cyclic monophosphate (cGMP). CNGCs are mediators of numerous effects of cyclic nucleotides in a plant cell [
74,
75]. CNGCs are involved in diverse signalling pathways ranging from plant development to stress responses, including pathogen responses and heavy metal homeostasis. Twenty AtCNGC genes have been found in
A. thaliana [
76].
Yeast mutants deficient in K
+ (
trk1 trk2) or Ca
2+ (
mid1 cch1) uptake systems have been used for functional characterization of some plant CNGCs. The mutant
trk1 trk2 helped to characterize the genes
AtCNGC1,
AtCNGC2 and
AtCNGC3 [
77,
78,
79,
80,
81]. It was demonstrated that the proteins encoded by the genes partially (compared to the protein AtKAT1) complement the yeast mutant under low K
+ concentrations.
An AtCNGC1 isoform was also functionally characterised in the yeast mutant
mid1 cch1 without two subunits of Ca
2+ channel complex Cch1/Mid1 in the yeast plasma membrane, which mediates high-affinity Ca
2+ influx and is involved in signal transduction in response to AMF (yeast alpha mating factor) [
72]. The exposure of such a mutant to AMF leads to growth arrest. The full-length protein AtCNGC1 did not complement this mutant in response to the pheromone, whereas the protein with the deleted C-terminus restored the wild-type phenotype. These results led to the conclusion that AtCNGC1 is permeable to Ca
2+ ions and that this permeability is regulated/inhibited by calmodulin interacting with the C-terminus of the protein [
77].
Evidence has been presented supporting the existence of weak voltage-dependent nonselective cation channels (NSCC), which are the main pathways for Na
+ entry into the roots at high soil NaCl concentrations [
82,
83]. CNGCs are among the subclasses of NSCCs that have been the subject of discussion in the context of Na
+ fluxes [
82,
84]. Two CNGCs from
A. thaliana, AtCNGC3 and AtCNGC10, have been linked to primary Na
+ fluxes in the roots [
75]. The Na
+-transporting function of AtCNGC3 was studied by the expression in the salt-sensitive
S. cerevisiae strain B31 (
ena1-4nha1), which lacks genes of the main transport systems for Na
+ export from the cytoplasm: the
ENA cluster and
NHA1 [
80]. The yeast mutant expressing AtCNGC3 was more sensitive to high salt concentrations; it also accumulated significantly more Na
+ than the control cells transformed by the empty vector. The authors concluded that AtCNGC3 forms a functional Na
+ permeable channel. The involvement of AtCNGC10 isoforms in Na
+ transport was also demonstrated by the heterologous expression in yeast strain B31 [
85].
The investigation of the mechanisms of absorption and regulation of Cl
− transport in plants is an important aspect of the study of plant salt resistance. Soil salinisation leads to the accumulation of not only Na
+ but also Cl
− in the plant cell cytoplasm at toxic levels [
86,
87]. The proteins of the CLC family (
Ch
Loride
Channel, family of anion channels and transporters) play an important role in the transport of Cl
− in prokaryotes and eukaryotes; members of this family participate in the transfer of not only Cl
− but also NO
3− [
88]. In addition to chloride channels, this family includes anion/proton exchangers, Cl
−/H
+- and NO
3−/H
+-antiporters.
In plants, CLCs play key roles in anionic homeostasis, salinity tolerance and nitrogen nutrition (for reviews, see [
89,
90,
91]). Plant CLC proteins are localised in endomembranes, where they perform many different functions: carrying out the electrogenic transport of NO
3− from the cytosol into vacuoles, regulating cytoplasmic concentrations of NO
3−, participating in the acidification of organelle lumens and regulating their transmembrane electric potential. Seven genes of the CLC family,
AtCLCa–e, have been cloned from
A. thaliana, and the functions and physiological roles of their products have been intensively investigated [
92].
To clarify the function of CLC genes, heterologous expression in the
S. cerevisiae mutant strain
Δgef1 is used (along with other approaches such as the patch-clamp technique and expression in Xenopus oocytes). In
Δgef1, the function of the only Cl
− channel/transporter, CLC homologue Gef1p, is distorted [
93]. The cells with the disrupted
GEF1 gene fail to grow on Fe-deficient medium with nonfermentable carbon sources; also, the
Δgef1 mutant cannot grow under alkaline conditions and demonstrates hypersensitivity to some extracellular cations (Na
+, Li
+ and Mn
2+) [
93]. Heterologous expression of the Cl
− transporter in the
Δgef1 mutant restores the growth of the mutant on appropriate selective media.
Δgef1 has also been successfully used for clarifying the anion selectivity of CLC proteins in diverse organisms. For example, it was shown that AtCLCc, AtCLCd and AtCLCf from
A. thaliana were able to complement the growth defect of the
Δgef1 yeast mutant, suggesting functional similarity with Gef1p [
92,
93,
94]. The expression of CLC genes from rice (
Oryza sativa),
OsCLC-1 and
OsCLC-2, in the
Δgef1 mutant demonstrated that both genes encode chloride channels [
95]. The same method was applied for the characterisation of Cl
− transporting CLC proteins from the moderately salt-tolerant
Glycine max and the salt-tolerant wild species
Glycine soja, namely, Cl
−/H
+ antiporter GmCLC1 and anionic channel GsCLCc2 [
96,
97].
Recently, the genes of anionic transporters of the CLC family from a salt-accumulating euhalophyte
Suaeda altissima have been cloned [
98,
99,
100,
101]:
SaCLCa1/a2 (the putative orthologs of
AtCLCa encoding NO
3−/H
+-antiporter of
A. thaliana [
102]),
SaCLCc1/c2 and
SaCLCd (the putative orthologs, correspondingly, of
AtCLCc and
AtCLCd encoding Cl
−/H
+- antiporters of
A. thaliana [
103,
104]), and
SaCLCf and
SaCLCg (the putative orthologs of
AtCLCf and
AtCLCg encoding Cl
− channels [
94,
105]). The CLC genes of
S. altissima were expressed in the mutant strain
Δgef1. This approach made it possible to characterise the functionality of the anionic carriers of
S. altissima. The experiments demonstrated that two of the identified proteins, SaCLCa1 and SaCLCa2, are NO
3−/H
+-antiporters, and the other five proteins are Cl
− transport proteins: Cl
−/H
+-antiporters (SaCLCc1, SaCLCc2 and SaCLCd) or chloride channels (SaCLCf and SaCLCg).
5. Investigation of Plant Ion Pumps Using Mutants of Saccharomyces cerevisiae
One of the early publications on the expression of plant proteins in yeast was the original work on the expression of the plasma membrane (PM) H
+-ATPase from
A. thaliana in
S. cerevisiae cells [
106]. Yeast cells contain their own PM H
+-ATPase with properties similar to those of the plant enzyme [
107]. Knockout mutations in the PM H
+-ATPase gene are lethal for yeast cells [
108]. Therefore, to avoid the co-existence of yeast ATPase and recombinant ATPase in the same cells, the authors used the yeast strain in which the constitutive promoter of the yeast plasma membrane H
+-ATPase gene
PMA1 was replaced by a galactose-dependent promoter
GAL1. This strain expressing yeast ATPase Pma1, which could grow on galactose medium but not on glucose medium, was transformed with a plasmid carrying the coding region of plant H
+-ATPase under the control of the yeast promoter
PMA1. The promoter
PMA1 conferred a high level of constitutive expression of the heterologous protein. The resulting transformant strain expressed endogenous yeast ATPase and heterologous plant ATPase in a galactose medium, and plant ATPase was expressed in a glucose medium as well. In these experiments, the recombinant protein did not reach the plasma membrane and accumulated in an intracellular membrane system (ER); nevertheless, this research is the first to demonstrate that functional plant plasma membrane H
+-ATPase can be synthesised in yeast cells in large amounts. In later experiments, using inducible promoters, which allowed independent control of the expression of the endogenous yeast H
+-ATPase and that of the heterologous pump, three isoforms of the
Arabidopsis pump, AHA1, AHA2 and AHA3, were expressed individually and their biochemical properties were characterised [
109].
The moss
Physcomitrella patens is salt-tolerant and able to grow at high concentrations of NaCl in a medium (up to 600 mM). This terrestrial plant has a gene encoding Na
+-ATPase, a transport enzyme that is absent in higher vascular plants [
20]. The function of the Na
+-ATPase PpENA1 from
P. patens was studied by heterologous expression in salt-sensitive
S. cerevisiae strain B31 [
20].
The functionality of some plant Ca
2+-ATPases has been studied in yeast mutants with defective endogenous Ca
2+ transport systems. In the yeast
S. cerevisiae, Ca
2+ homeostasis is maintained by P-type Ca
2+-ATPases, Pmc1 (vacuolar Ca
2+ pump), Pmr1 (Ca
2+ pump of Golgi apparatus) and the vacuolar Ca
2+/H
+ antiporter Vcx1, which is inhibited by calcineurin and is not active at low Ca
2+ concentrations [
110]. In the yeast strain K616 (
pmr1 pmc1 cnb1) [
111], Pmc1, Pmr1 and the regulatory calcineurin subunit Cnb1, which is involved in controlling the activity of the yeast vacuolar Ca
2+/H
+ antiporter, are deleted, and the maintenance of Ca
2+ homeostasis relies solely on Vcx1 activity. At physiological Ca
2+ concentrations (≥1 mM Ca
2+), the mutant grows as well as the WT, but growth is inhibited at low Ca
2+ concentrations because of the inactivation of Vcx1, and complementation assays in the K616 strain are used for the functional analysis of Ca
2+-ATPases [
112]. Several types of IIA and IIB Ca
2+-ATPases have been heterologously expressed in yeast mutants [
16,
113,
114].
The function of the Ca
2+-ATPase ECA1 from the endoplasmic reticulum of
A. thaliana was characterised in yeast mutants defective in the Ca
2+ pump of the Golgi apparatus (mutant
pmr1) or in both the Ca
2+ pumps of the Golgi apparatus and the vacuolar Ca
2+ pump (mutant
pmr1 pmc1 cnb1) [
16].
In the K616 (
pmr1 pmc1 cnb1) strain, auto-inhibited Ca
2+-ATPases from
Arabidopsis, isoform 2 (ACA2) and isoform 4 (ACA4), a calmodulin-regulated Ca
2+-ATPase, were characterised [
113,
114]. Calmodulin-binding studies and complementation experiments demonstrated that the N-termini of ACA2 and ACA4 contain an auto-inhibitory domain with a binding site for calmodulin. ACA4, as well as Pmc1, the yeast vacuolar Ca
2+-ATPase, conferred protection against osmotic stresses such as high NaCl, KCl and mannitol when expressed in the K616 strain. An N-terminally modified form of ACA4 specifically conferred increased NaCl tolerance, whereas full-length ATPase had less effect [
114].
Yeast mutants defective in endogenous Ca
2+ transport systems were used for expression and functional studies of the Ca
2+-ATPase PpPCA1 from
P. patens [
115]. These studies demonstrated the presence of an autoinhibitory N-terminal domain in PpPCA1.
Vacuolar H
+-pyrophosphatase functions in the tonoplast of plant cells to transport protons across the membrane at the expense of the energy released from the hydrolysis of inorganic pyrophosphate (PP
i). Apart from the tonoplast, this enzyme is also found in the membranes of the Golgi apparatus. Thus, inorganic pyrophosphatase plays an important role in the regulation of vacuolar pH and also in the control of vesicular transport in plant cells [
116]. Pyrophosphatase AVP1 from
A. thaliana was expressed in the
vma1 mutant of
S. cerevisiae, which was defective in the activity of V-type H
+-ATPase and the H
+-transporting activity of the studied heterologous enzyme [
117]. Inorganic pyrophosphatase from
A. thaliana complemented the transport function of yeast vacuolar H
+-ATPase, thus confirming the ability of H
+-pyrophosphatase to create a physiologically significant pH gradient at the vacuolar membrane.
6. Systems of Heterologous Expression and Research on Function of Mutant Protein Forms
Systems of heterologous expression help to elucidate the structure and functions of mutant protein forms.
Mutant forms of
Arabidopsis KAT1, a hyperpolarisation-activated K
+ channel expressed mainly in the stomatal guard cells, were investigated by a combination of random site-directed mutagenesis and screening of a
trk1 trk2 yeast strain transformed with the mutant KAT1 forms [
118]. Strong modifications in the cation selectivity of the KAT1 mutant forms were revealed in this study. A sensitive site (T256) in the pore domain of the channel was found; mutations at this site significantly affected the cationic specificity of the channel.
High-affinity plant K
+ transporters HAK together with inward rectifying K
+ channels AKT1 carry the main contribution of ion uptake by roots; moreover, at low external K
+ concentrations, the uptake of K
+ is likely to occur in symport with H
+ [
119]. It was shown for the AtHAK5 transporter from
A. thaliana that the mechanism is the only one for K
+ uptake at extremely low (below 10 μM) external K
+ concentrations [
120,
121]. The role of the transporter HAK5 is important under saline conditions [
122]. Research by Aleman et al. [
123] used heterologous expression of mutant HAK5 forms from
A. thaliana in
S. cerevisiae cells, allowing identification of the point mutation in
AtHAK5, which significantly increased the salt tolerance of yeast cells. The mutation at amino acid residue F130, which is highly conservative for HAK5 proteins from different species increased more than 100-fold the affinity of the transporter to K
+ and decreased its affinity to Na
+. The authors concluded that the amino acid residue F130 contributes to the formation of a selectivity filter of the
AtHAK5 transporter.
7. S. cerevisiae Serves as a Toolkit to Study Protein–Protein Interactions
Strains of baker’s yeast are used not only to identify the genes of ion transporters and ascertain the selectivity of the transporters in experiments with functional complementation of yeast mutants but also to understand protein–protein interactions. This important application of yeast mutants is aimed at finding the potential regulators that determine the activity of ion-transporting proteins.
Among the methods available for studying protein–protein interactions in vivo are Y2H (yeast two-hybrid), FRET (Förster resonance energy transfer), BiFC (bimolecular fluorescence complementation) and SLCA (split luciferase) (reviewed in [
124]), but the techniques using heterologous expression in yeast cells remain, apparently, the most accessible and inexpensive. Already a classic approach to the detection of protein-protein interactions is the yeast two-hybrid system (Y2H, Yeast two Hybrid) [
125,
126]. Since its description in 1989 [
127], this system has been widely used to identify protein–protein interactions in many organisms.
The heterologous expression of a single recombinant protein (protein of interest, POI) sometimes does not produce any distinct phenotypes under selective conditions. One of the reasons could be that it requires additional regulatory proteins, so the co-expression of the POI together with its regulators may solve this problem [
124,
125,
126]. Y2H is based on the fact that many eukaryotic transcription factors include two functionally different domains, one of which ensures DNA binding and the other one controls the activation of transcription. For a classic operation of the technique [
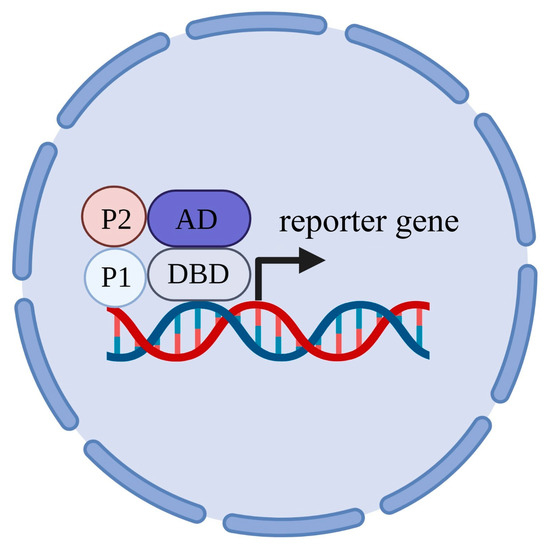
127], POI (so-called “bait”) is fused to the DNA binding domain of a transcription factor, which regulates the transcription of the reporter gene. The other protein under the study (“prey”) is fused to the activation domain, which is required to activate the transcription of the reporter gene by RNA-polymerase II. The physical interaction of the two studied proteins leads to the physical association of the DNA-binding domain with the activation domain of the transcription factor, restores its function and activates the transcription of the reporter gene (
Figure 4). Genes
HIS3 and
ADE2 are often chosen as the reporter genes; these genes are required for the biosynthesis of histidine and adenine and are able to rescue the growth of histidine auxotrophic and adenine auxotrophic yeast strains, correspondingly, in media deficient in these compounds.
Figure 4. Scheme of classic yeast two-hybrid technique. DBD—DNA-binding domain; AD—activation domain; P1—“bait” (protein of interest, POI); P2—“prey”.
The accustomed way of using the yeast two-hybrid system has essential disadvantages [
126]. For example, there is a high probability of false-positive or false-negative results, which could be a consequence of direct activation or inhibition of the heterologous proteins by themselves with the transcription of the reporter gene. Furthermore, the traditional yeast two-hybrid system is applicable for interactions between soluble proteins that could be transported to the nucleus; however, this system is not applicable for detecting protein–protein interactions for membrane proteins. At present, several modifications of the traditional yeast two-hybrid system have been developed that provide opportunities to overcome most of the abovementioned limitations of the system [
128,
129,
130,
131,
132,
133,
134].
One of the modifications of the yeast two-hybrid analysis was adapted for the investigation of protein–protein interactions with the participation of membrane proteins. This method is based on the recovery of functional ubiquitin, the so-called split-ubiquitin Y2H system [
126,
131,
132]. The protein used in this study is fused with the C-terminal fragment of ubiquitin and with the transcription factor that controls the expression of the reporter gene. The other protein used in this study is fused with the modified N-terminal fragment of ubiquitin with an amino acid substitution I13G, which renders this ubiquitin fragment low affinity to the C-terminal ubiquitin fragment (
Figure 5). Since the transcription factor is linked by covalent bonds to the membrane protein and is localised outside of the nucleus, it is not able to activate the transcription from the reporter gene. However, when the two heterologous proteins in this study interact, the N- and C-terminal fragments of ubiquitin merge; the complete ubiquitin is recognised by the ubiquitin-specific proteases and finally the transcription factor is released. The transcription factor can then be transported to the nucleus and activate the transcription of the reporter gene (
Figure 5).
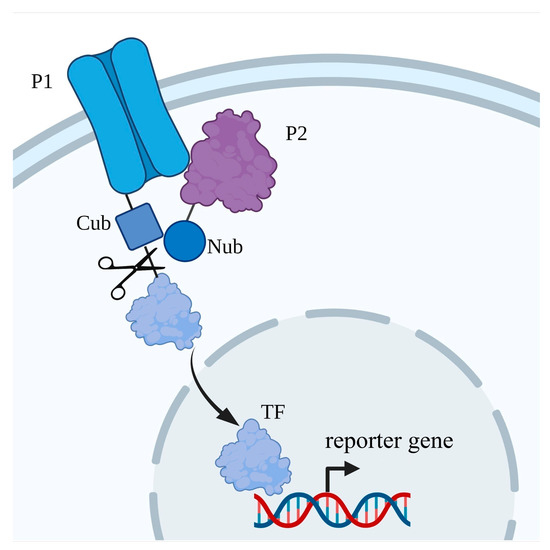
Figure 5. Scheme of split-ubiquitin Y2H system. P1—“bait” (protein of interest, POI), fused with C-end of ubiquitin (Cub); P2—“prey”, fused with N-end of ubiquitin (Nub); TF—transcription factor.
Y2H as a powerful technique for the study of protein–protein interactions can be used to search for novel interacting partners by screening a single protein of interest or domain against a library of other proteins (i.e., libraries of genomic DNA, cDNA and ORF). The complete DNA library carries the prey element (prey library), and the POI is cloned into a plasmid as bait (bait plasmid). Commercial kits are available for performing such tasks, including the membrane-based yeast two-hybrid system, allowing the use of full-length integral membrane proteins and membrane-associated proteins as baits to hunt for unknown interaction partners.
8. Protein–Protein Interaction with Ion Transporters of Plants That Were Discovered in S. cerevisiae
The abovementioned approaches were used to identify the proteins interacting with potassium and sodium transporters in plants (reviewed in [
30]). For potassium transporters, the experiments revealed protein regulators for HKT transporters and shaker-type potassium channels. Different protein kinases, protein phosphatases, calcium sensors, GTPases, syntaxins and anion channels were identified among the proteins that influence the transport activity of potassium channels. Moreover, the phenomenon of homo- and hetero-oligomerisation was discovered for several transporters, such as AKT1/2, KAT1 (inward rectifiers), GORK and SKOR (voltage-gated outward rectifying K
+-channels that allow K
+ passage out of cells and open under membrane depolarisation (comprehensively reviewed in [
135]).
The SOS system for the removal of excessive Na
+ from plant cells is a good example where the expression of a single heterologous protein in yeast did not lead to a distinct phenotype under selective conditions but required additional components to activate the ion-transporting protein.
SOS genes (
Salt Overly Sensitive) were identified when screening mutants of
A. thaliana with hypersensitivity to NaCl [
136]. These genes were classified into three groups:
SOS1,
SOS2 and
SOS3. The genome of
A. thaliana has one
SOS1 gene, 24
SOS2 genes and 9
SOS3 genes [
137]. The SOS1 protein is a Na
+/H
+-antiporter in the plasma membrane, which is important for keeping low cytoplasmic Na
+ concentrations. SOS1 proved to be one of the main determinants of salinity tolerance in plants [
138]. SOS2 proteins are serine/threonine protein kinases that activate SOS1 by phosphorylating its C-terminal domain. In turn, the functioning of SOS2 proteins requires that they form a protein complex with the calcium-binding protein SOS3, which belongs to the family of calcineurin B-like proteins (CBLs). Therefore, the heterologous expression of SOS1 alone could not completely complement the salt-sensitive
S. cerevisiae mutant B31 (
ena1-4 nha1); the co-expression of
SOS1 with
SOS2 and
SOS3 essentially increased the salt tolerance of the B31 strain [
139].
Several ion channels, including the inward-rectifying K
+ channel KAT1, play a key role in the opening of stomata [
140,
141]. With KAT1 from
A. thaliana, it was shown that this channel can interact with protein OST1 (open stomata 1) [
142]. Protein OST1 (also known as SnRK2.6) is a stress-induced protein kinase that is involved in ABA signal transduction. The interaction between OST1 and KAT1 results in negative regulation of the ion channel (probably due to its phosphorylation), so the potassium fluxes into the guard cells decrease the stomatal aperture and water losses by the plant [
142].
Among the many regulators of KAT1 is BCL2-associated athanogene4 (BAG4) (reviewed in [
30]), which was revealed using the yeast split-ubiquitin system. BAG4 interacts with KAT1 and favours the trafficking of KAT1 to the plasma membrane. Two
Arabidopsis mutant lines without the
BAG 4 gene exhibited a delay in the stomatal opening ([
30] with references therein on several other regulators of KAT1).
A study of the model plant
A. thaliana revealed a complex regulatory network in plant roots where the inward-rectifying potassium channel AKT1, which is responsible for potassium uptake from the medium, is gated (regulated) by Ca
2+-dependent proteins. The interaction of calcineurin B-like protein (CBL, sensor of calcium) with CBL-interacting protein kinase 23 (CIPK23) led to the formation of an active protein complex that could bind to the C-terminal domain of the ion channel AKT1 [
143]. Further phosphorylation of AKT1 by kinase CIPK23 activates the ion channel. Another participant of the regulatory network that interacts with AKT1 is a protein phosphatase of the 2C family (PP2C), AIP1 (AKT1-interacting PP2C1). The dephosphorylation of AKT1 by AIP1 inactivates K
+ ion currents via AKT1. Deciphering the network for AKT1 regulation started with the aid of yeast two-hybrid systems and continued using
A. thaliana mutants and other heterologous systems such as Xenopus oocytes for electrophysiological recordings [
143,
144]. Interestingly, transcripts of CBL and CIPK23 from
A. thaliana restored the functions of the heterologously expressed AKT1 from barley [
145], providing evidence for cross-species complementation in the system. Later development of the yeast two-hybrid system added more components (a silent K
+ channel, KC1) to the AKT1 regulatory network [
146].
This entry is adapted from the peer-reviewed paper 10.3390/ijms241310768