Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
5-hydroxymethylfurfural (HMF), which can be synthesized from lignocellulose as a raw material, is an important biological platform molecule. Its preparation and the catalytic oxidation of subsequent products have important research significance and practical value. In the actual production process, porous organic polymer (POP) catalysts are highly suitable for biomass catalytic conversion due to their high efficiency, low cost, good designability, and environmentally friendly features.
- porous organic polymer
- biomass conversion
- lignocellulose
- 5-hydroxymethylfurfural
1. Introduction
Facing the current global energy and environmental problems, it is urgent to replace fossil energy with renewable and environmental alternatives to produce a series of high-value chemicals and clean fuels. The effect of biomass resources on these two aspects can effectively overcome the dependence on fossil energy [1][2]. Lignocellulose, the main structural component of plants, is by far the most abundant renewable resource. It is capable of being converted in significant quantities into biofuels, such as bioethanol [3], and other commercial chemicals, such as 5-hydroxymethylfurfural (HMF), sugars, and phenols.
HMF is a biological platform molecule that can be produced from lignocellulose, that is, lignocellulose is hydrolyzed into six-carbon sugars (glucose and fructose) and further dehydrated [4]. It is an intermediate as well as a raw material for many important chemicals and can be converted into high value-added chemicals by oxidation, hydrogenation, polymerization, and ring-opening reactions by its own furan rings, aldehyde groups, and hydroxyl groups. Some of the products from HMF such as cross-linking agents of polyvinyl alcohol (PVA) for battery manufacturing [5] and γ-valerolactone (GVL), which produces high value-added fuels. In particular, 2, 5-furanodicarboxylic acid (FDCA), for the substitution of petroleum-based aromatic compounds, can be produced by the oxidation path of HMF [6]. It can be polymerized with ethylene glycol to produce polyrthylene furandicarboxylate (PEF), which can replace polyethylene terephthalate (PET) synthesized from petroleum-based compounds as precursors [7]. Furthermore, other polyester products synthesized by FDCA are also promising, such as bio-based copolyesters, which can be used as 3D printing materials [8].
The existing catalytic preparation of HMF can be roughly divided into two categories. Homogeneous catalysts are represented by inorganic acid [9], ionic liquid [10], metal salt [11], etc. In this kind of catalyst system, although the active center of the catalyst is uniform and the structure is clear, the separation of the catalyst in the reaction mixture is difficult and may be accompanied by side reactions, and the recovery performance of the catalyst is greatly reduced. Consequently, from an industrial perspective of product separation and equipment maintenance, etc., a heterogeneous catalyst is more advantageous. Due to various defects and the local environment of active sites, the coexisting environment of multiple active sites created is beneficial to improve the catalytic efficiency, but attention should also be paid to product selectivity. Some researchers have used metal oxides [12], metal carbide [13], functional carbon materials [14], etc., as catalysts for achieving HMF.
Supported catalysts, as typical heterogeneous catalysts, have naturally become a hot option for catalyst structures, and can not only be combined with precious metals to further improve their efficiency but can also achieve efficient conversion by combining non-precious metals [15] or even environmentally friendly non-metallic components [16] with suitable carriers. Here, the selection of the catalyst support and active ingredients (sites) are very important. For the support, it is often a class of a solid porous material with a high surface area, suitable pore size and distribution, and adequate pore volume. Of course, some porous supports can also be directly used as the catalyst or cocatalyst themselves [17].
A porous material is a kind of functional material composed of interconnected or closed holes. According to the pore size, they can be divided into micropore materials (<2 nm), mesoporous materials (2–50 nm), and macropore materials (>50 nm). We are familiar with porous materials such as metal-organic frameworks (MOFs), porous organic polymers (POPs), activated carbon, silica, clay, and so on [18]. They have been used in a wide range of fields, nowadays, such as porous silicon for the manufacture of photoelectric components, porous membranes, and porous adsorbents for separation, thermal, and sound insulation materials, biomedical materials, and so on [19]. Porous materials can not only improve the utilization of the volume of material based on the stability and screening characteristics of the pores but also provide more space for the contact between active sites and reaction substrates, which greatly promotes the performance of catalysts [20]. When metal components are the important active sites, a supported porous catalyst can ensure high efficiency, but there are also side reactions, low selectivity, and metal leaching into the product [21]. Hence, as one of the hot topics in catalysis research, nonmetallic porous carbon materials have the advantages of low density, no metal leakage pollution, good structural ductility, and strong acid and alkali resistance [22]. They are often used as a kind of catalytic material with great potential for energy storage and conversion [23] and can realize efficient conversion through the functionalization and loading of active components. They have excellent application in catalytic biomass valorization and environmental remediation [24]. However, since porous carbon materials are usually prepared by high-temperature pyrolysis, it is relatively difficult to predict and control their structural features [25].
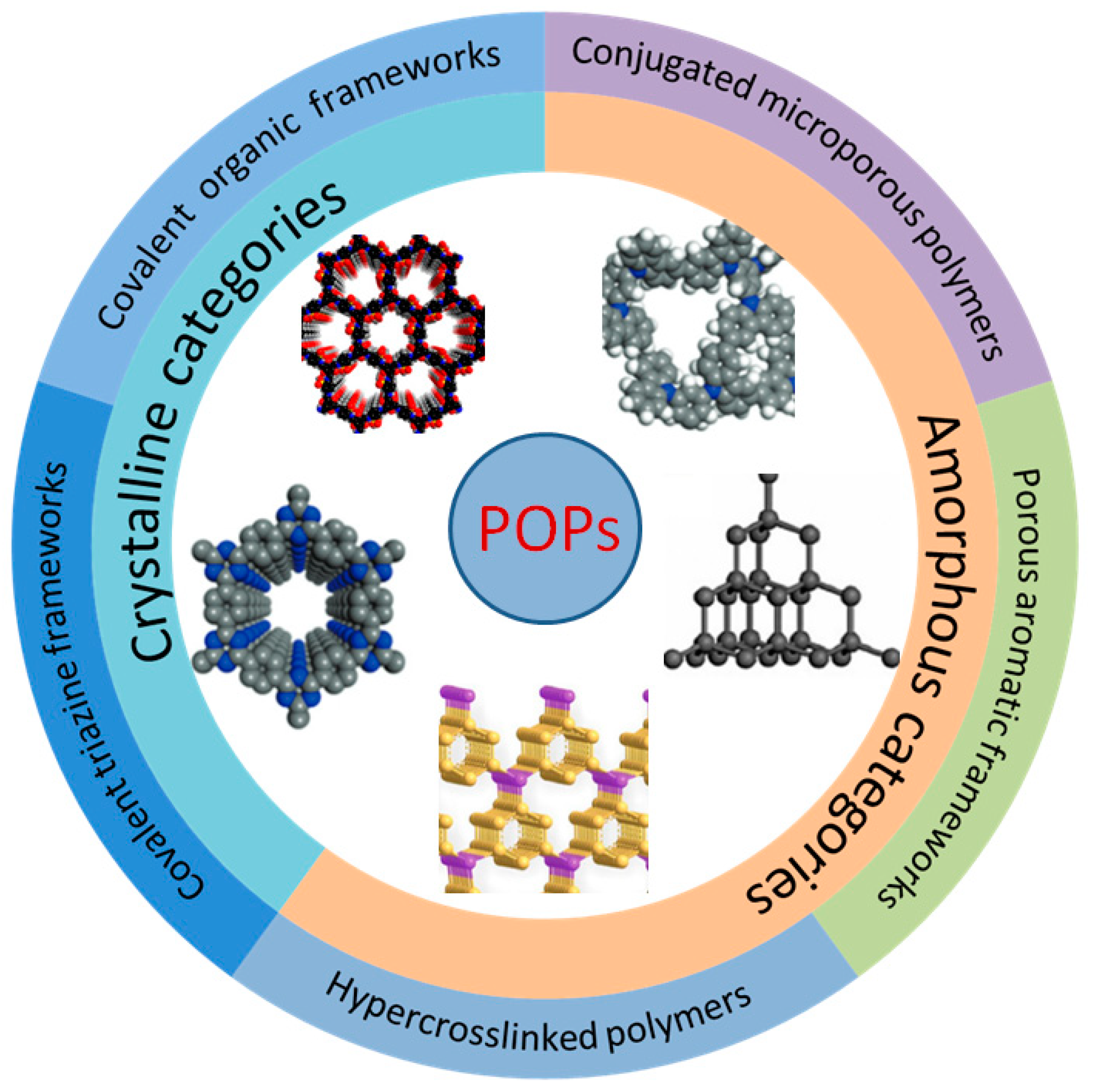
2. Synthesis and Structure of POPs
A POP is a kind of organic porous functional material with a microporous and mesoporous structure. As shown in Figure 1, according to their structural characteristics, they can be divided into amorphous categories (representing PAFs, CMPs, and HCPs) and crystalline categories (representing COFs and covalent triazine frameworks (CTFs) [26]). POPs can be classified into different categories and achieve various functions based on different monomers and synthesis routes, such as Suzuki coupling [27], the Friedel–Crafts alkylation reaction [28], Schiff-base condensation [29], and Hagihara cross-coupling [30], etc. Their unique physical and chemical properties have enabled POPs to achieve good results in adsorption, catalysis, sensing, battery production, energy storage, biomedicine, film material, and other fields [31][32][33][34][35].

Figure 1. Partial classification of POPs.
COFs are emerging crystalline porous molecular materials composed of organic molecules linked by strong covalent bonds (such as B-O, C=N, C-N, C=C, etc.) and are composed of two main components: the covalent bond skeleton and the modifiable functional group. COFs were first synthesized and reported by Omar Yaghi et al. in 2005 [36]. On account of the large specific surface area, periodicity, and regular and adjustable channel structure, the functional surface and the inner wall of the channel can be used for catalysis after loading the active component. From the perspective of topological structure, COFs can be divided into two categories. Two-dimensional COFs have a unique, layered morphology, namely conjugate planes and π-π structures stacked between layers, which form regular channels in the longitudinal direction. Three-dimensional COFs are connected by covalent bonds to form a network, so they are highlighted by many open sites and low density [37]. Both structures can ensure the stability of COF crystallinity by placing active sites into their own structures, which is also very representative of heterogeneous catalysis. The polymerization of COFs is generally carried out according to the principles of dynamic covalent chemistry and reticular chemistry, and the final product is determined by the thermodynamic stability of the substrate. However, due to their dependence on dynamic reversible reaction synthesis, their stability is lower than that of other POP subclasses, and due to the limitations of reversible reaction types, their synthesis scheme and structural diversification need to be developed.
Unlike COFs, PAFs are usually formed through irreversible coupling reactions, such as Ullmann coupling. The term PAFs was first proposed in 2009 [38]. A diamond-like structure was formed by a rigid benzene ring, as shown in Figure 1. Interestingly, in addition to a large internal surface area, a very strong skeleton formed by a bottom-up C-C bond between aromatic structural units enabled excellent structural stability even under some extreme conditions [39]. In summary, there are three important aspects that determine the synthetic diversity and performance of PAF materials, which are symmetrical structure-containing monomers, efficient coupling reactions, and the topological design of the framework. By modifying the functional group of the framework unit, the specific function of a certain substance can be realized. For example, the capture of CO2 can be realized through the amino group on the aromatic structure [40]. At the same time, its adjustable physical and chemical properties and large specific surface area can also be well used in biomass catalysis.
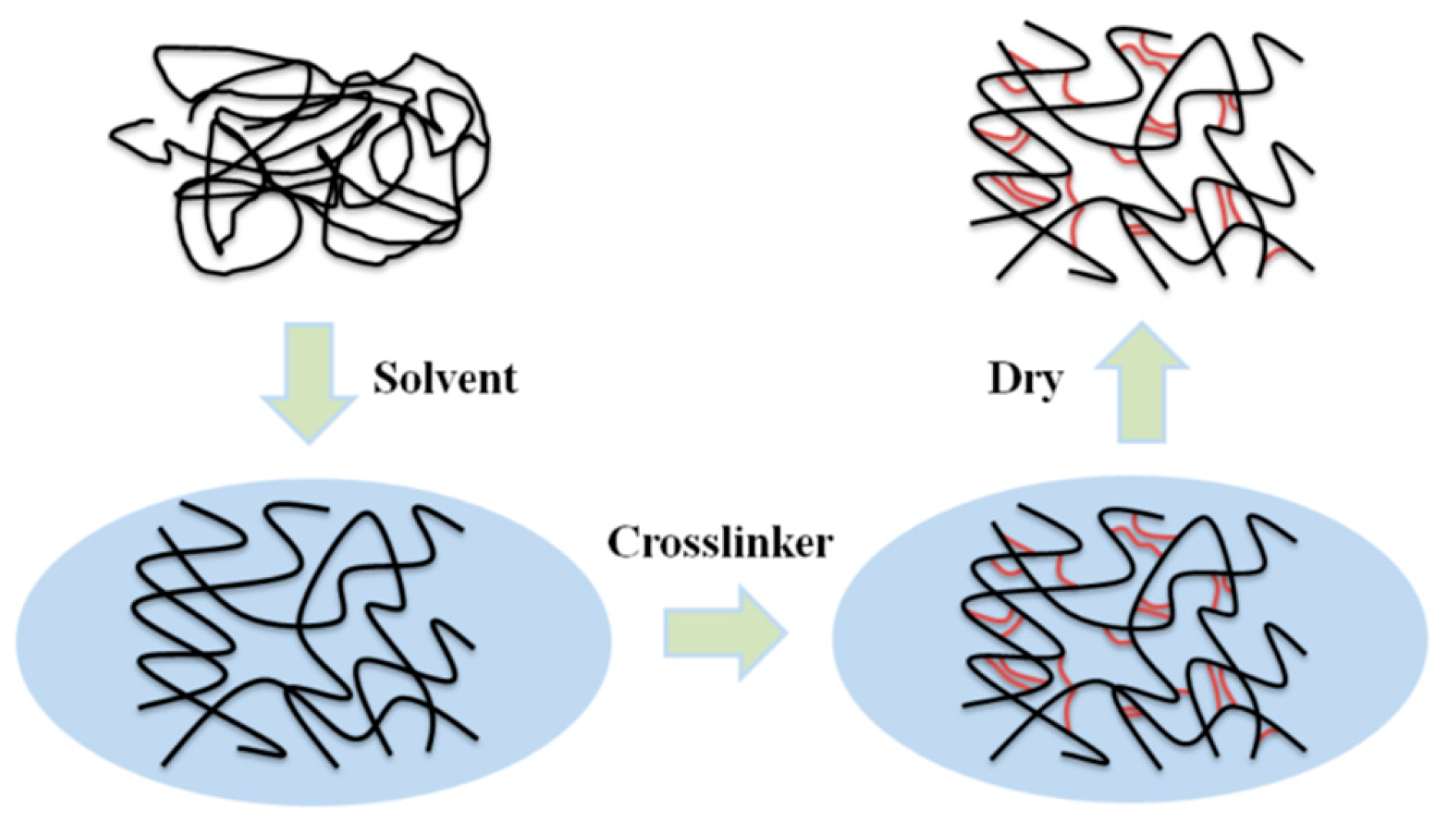
Porous materials with good structural properties often have a higher preparation cost, but HCPs differ from other types of POPs in that they can be directly synthesized by post-modification easily through Friedel–Crafts (FC) reactions in mild conditions [41]. As a kind of polymer material with a permanent microporous structure, HCPs can achieve a specific structure depending on the choice of monomer, cross-linking agent, and appropriate reaction routes. Figure 2 shows the specific hyper-cross-linked process. At present, there are three main synthesis methods: post-cross-linking [42], direct polycondensation [43], and external cross-linking [44]. Their large surface area, light weight, and mild prepared condition make HCPs particularly outstanding in the fields of adsorption and catalysis.

Figure 2. Schematic diagram of hyper-cross-linked process.
3. Catalytic Conversion of Fructose on POPs
Natural wood after a simple treatment can become non-toxic, tasteless, and pollution-free lignocellulose, and the use of biomass resources is mostly concentrated on the treatment [45]. The hydrolysis of cellulose into glucose is the starting point of its high-value transformation. Glucose can not only produce the most widely used industrial raw material ethanol, but also isomerize to fructose for producing lactic acid, HMF, and other important platform compounds. Hemicellulose can also be converted into furfural, xylitol, and other compounds after acid hydrolysis. Lignin is the most abundant renewable aromatic compound in nature and can also be used in the production of fuel and high-value chemicals. How to overcome the complexity and obstinacy of its structure and realize its efficient utilization is necessary to continue to study. Using efficient catalytic technology to transform lignocellulose into high value-added chemical platform compounds and derive downstream green chemicals on the basis of platform molecules will accelerate the arrival of the era of carbon neutrality.
At present, researchers have used cellulose as a raw material to prepare HMF by a one-pot method [46] or a multi-step method, and others have also used mannose with the same intermediate as glucose as raw materials and other polysaccharides for conversion [47]. One is directly preparing it by removing three molecules of water from glucose. The second is by using fructose as a starting point under certain conditions to generate a specific intermediate and gradually removing three molecules of water collocation [48]. Wang et al. proposed the mechanism of C6 dehydration to prepare HMF, and reasonably concluded that the precondition for glucose to HMF was to convert it into fructose first [49]. Structurally, glucose with a pyran ring structure was more stable and more difficult to convert into HMF than fructose with a furan ring structure [50]. In a specific study on the preparation of HMF from lignocellulose-derived fructose, the results and analysis also verified the reliability of the above transformation mechanism [51]. If the efficient catalytic technology can be used to convert biomass resources into high value-added chemical platform compounds, and these platform molecules can derive downstream green chemicals, this will accelerate the arrival of the era of carbon neutrality [52].
In a reaction system with POPs as the main component of the catalyst, strong covalent bonds were created between the monomers to ensure the stability of the structure in a solvent, ensuring that the substrate could fully contact the active site in the catalytic reaction [53]. After multifunctional acid functionalization, it had corresponding groups, which ensured high conversion and selectivity. The subclasses of POPs have gained favor among scientists. For example, regarding amorphous PAFs and HCPs, the former has an extremely high surface area, while the latter has economical and mild synthesis conditions, both of which have achieved extremely high conversion rates in biomass conversion, especially fructose.
Due to the fact that most COFs are synthesized by reversible reactions, they are not only less stable than other POP types but also limited to the symmetry of the structural monomers. Therefore, they need to be developed to improve their synthesis scheme and topological structure to improve their stability [54]. Peng et al. prepared irreversible enol-ketone intervariant structures with significant chemical stability by an alkali condensation reaction and treated with 1, 3, 5-triformyl-phloroglucinol (TFP) and 2, 5-diaminobenzene sulfonic acid (DABA) to obtain a solid acid catalyst, called TFP-DABA [55].
The synthesis system of COFs endowed them with unique structural properties. In the formation and connection of the covalent bonds, the high synthesis rate sped up the nucleation process in the synthetic system, which led to a great reduction in the crystallinity of the COFs, and made it difficult to obtain single-crystal COFs. This structure made their stability weaker than that of other subclasses. This characteristic was reflected in the catalytic reaction, which shortened the catalyst life and affected the economic and time benefits [56].
In addition to increasing the specific surface area and the number of acid sites of the catalyst, the catalytic activity and product selectivity of converting fructose into HMF was also considered due to the low diffusion of fructose molecules in the cellular structure [57]. Subsequently, some PAFs were recently reported for this catalytic reaction. Du et al. used rigid tetrahedrons and triangles to construct porous aromatic framework PAFs to ensure the suitable pore size and the availability of active sites [58]. Their catalyst HO3S-POP was highly adapted to the substrate with a high specific surface area and could achieve a 100% fructose conversion within 15 min under microwave heating to 140 °C, and 70% HMF selectivity.
Unlike crystalline framework materials, such as COFs, PAFs are composed of irreversible structures, more attention should be paid to the uniqueness of their topological design. The structure formed by an appropriate coupling reaction can realize high product selectivity. In the local structure, aromatic groups with certain rules can rotate and combine to deform, thus forming structural defects that fit with the reaction system. Therefore, it is necessary to clearly study the framework of PAFs, understand their structural composition in depth, and further broaden the scope of their heterogeneous catalysis.
Although HCPs with a permanent microporous structure cannot be dominant in fructose diffusion, the preparation and application of HCPs with a low threshold is still one of the best choices for porous catalytic materials. Das et al. synthesized the microporous polymer TrzDBTH by the Friedel–Crafts reaction of 2,4,6-tris [4-(bromomethyl)phenyl]-1,3,5-triazine and dibenzothiophene (DBTH), and then sulfonated it with chlorosulfonic acid to obtain a solid acid catalyst, STrzDBTH, with sulfonic acid groups [59]. The complete conversion of fructose and 96.2% yields of HMF were achieved after a 20 min reaction at 140 °C under atmospheric pressure. The catalyst could maintain five reaction cycles. Dong et al. verified the effect of the sulfonation treatment and reaction solvent on the dehydration of fructose into HMF by the self-polymerization of a benzene monomer into HCP-x [60].
Sulfonic acid-functionalized materials are widely used in the field of catalysis [61]. In the dehydration reaction of the fructose preparation of HMF, a POP catalyst was almost sulfonated in the synthesis process and contained sulfonic acid sites, which proved able to improve the reaction efficiency. To broaden the research direction, Ravi et al. [62] used phosphate-functionalized POPs for biomass conversion into HMF for the first time. They synthesized the HCP-structured catalyst (B-POP) by replacing the sulfonic acid site with a phosphoric acid site. The reaction was carried out in DMSO at 160 °C for 30 min to obtain a 100% fructose conversion and 85% HMF production. The catalytic effect was further used to convert HMF into the oxidation product DFF and the ring-opening product Levulinic acid (LA).
4. Catalytic Conversion of HMF on POPs
Climate change and the growing depletion of non-renewable energy are putting enormous pressure on all sectors of the world, forcing economies to scale up to meet demand [63]. The chemical industry, which relies on fossil fuels to run, urgently needs to change this status. As mentioned above, PEF using FDCA as the precursor has the potential to replace PET, but the current process route for FDCA production is not mature, and it has not been able to achieve large-scale production of degradable polyester for practical application [64]. The most possible method for large-scale production is to prepare FDCA by the catalytic reaction of HMF, which can be converted from biomass resources. For different oxidation methods, the catalytic methods for preparing FDCA from HMF can be divided into thermal catalysis [65], photocatalysis [66], electrocatalysis [67], and biocatalysis [68]. According to the current production level, thermal catalysis is the most compatible method in industry.
Two-dimensional COFs were designed from symmetric and asymmetric topologies to order layered stacked lattice patterns, and regular carrier channels formed along the longitudinal axis, so there is also space for application in the field of electrocatalysis [69]. Since most COFs are non-conductive materials, in order to improve their catalytic efficiency, their unique structures could be used to carbonize the pyrolysis treatment template, and their utilization forms could also be efficiently designed. Cai et al. used nickel acetate, triformyl-phloroglucinol-5,5-diamino-2,2-bipyridine, and toluene sulfonic acid to obtain 2D COF structure-containing films by interfacial crystallization and stamped them onto fluorine-doped tin oxide (FTO) to prepare the catalyst TpBpy-Ni@FTO [70]. This achieved up to a 96% HMF conversion and obtained 58% and 34% FDCA and FFCA oxidation products, respectively. As the first case of electrocatalytic oxidation of HMF with a COF structure, the product yield of this system was not ideal.
FDCA is not the only important substance in the oxidation path of HMF. Others such as DFF, 5-hydroxymethyl-2-furan carboxylic acid (HMFCA), and 5-formyl-2-furan carboxylic acid (FFCA) can also be widely used in the preparation of fine chemicals, pharmaceuticals, chiral catalysis, and polyester industry [71][72][73]. Similarly, the oxidation route is not the only one worth discussing in the utilization of HMF. HMF, known as “the sleeping giant” [74], can activate C=O, C=C, and C-O itself for high-value conversion in green sustainable chemistry. 2,5-dihydroxymethylfuran (DHMF) is an important chemical in the production of artificial fibers and resins and can be produced by the hydrogenation of HMF [75]. Most studies have relied on the synergistic system of Cu0 and Cu+ to improve the catalytic activity in hydrogenation treatment [76]. However, due to the existence of reducing gases, the stability of the system needs to be strengthened [77]. Therefore, it has been very important to develop a stable structure of a Cu-supported catalyst in this direction.
In particular, 5-ethoxymethylfurfural (EMF) was identified as an important substance for the preparation of biodiesel and can produce a change from the currently used petroleum-based energy systems [78]. Wang et al. used the soft template method to synthesize ordered mesoporous carbon (OMC) from resorcinol and pluronic F-127, and then sulfonated it to produce a catalyst, OMC-SO3H. The fructose was converted into EMF with a yield of 55.7% by a one-pot method [79]. On this basis, Zhang et al., used sulfonic acid functionalized HCPs instead of OMC as the catalyst for the direct reaction of the one-pot method into a mixed solvent system of ethanol and DMSO and obtained EMF and HMF with yields of 78.9% and 15.4%, respectively, by reaction at 104.85 °C for 480 min. The activity of the catalyst HCP-x did not decrease significantly after five catalytic cycles [80]. In this reaction, the high-density sulfonic acid groups and the stability of HCP-based catalysts ensured the activity of fructose etherification. Its abundant Brϕnsted acidic site and high mobility given by its structure guaranteed the efficient conversion of the product with the addition of Lewis acid.
In addition to the functional modification of POPs, supported catalysts prepared with active components achieved higher performance than the catalysts composed of only active components. In the utilization of PDVTA, Lai et al. loaded Cu-doped MnO2 nanowires to prepare Cu-MnO2@PDVTA, which achieved up to a 96.8% FDCA yield at 80 °C for 12 h, and the catalyst achieved seven cycles [81].
In addition to performing functional modifications, POPs can achieve different functions through the composition of monomers. For example, in order to improve the adsorption capacity of CO2, Modak et al. used porphyrinic molecules to react with phenyldialdehyde and synthesized the materials with a surface area of 875 m2/g through a one-pot method [82]. Saha et al. used this strategy in the preparation of a catalyst for HMF conversion. They used pyrrole and phenylaldehydes after distillation as precursors, and added ferric chloride to acetic acid mediated through hydrothermal synthesis to obtain an FeIII-POP-1 catalyst. In this reaction, 100% conversion of HMF and a 79% yield of FDCA could be obtained by using only water as a solvent and reacting in air at 100 °C for 10 h. The catalyst maintained activity in three reaction cycles [83]. No product appeared in the system after the subsequent reaction with a homogeneous FeIII–porphyrin complex, which indicates the importance of a large surface area and the multiple active sites produced by this simple and rapid synthesis method for catalytic reaction, and also reflects the interoperability of the two fields of adsorption and catalysis.
This entry is adapted from the peer-reviewed paper 10.3390/polym15122630
References
- Bender, T.A.; Dabrowski, J.A.; Gagné, M.R. Homogeneous catalysis for the production of low-volume, high-value chemicals from biomass. Nat. Rev. Chem. 2018, 2, 35–46.
- Lange, J.-P.; Price, R.; Ayoub, P.M.; Louis, J.; Petrus, L.; Clarke, L.; Gosselink, H. Valeric Biofuels: A Platform of Cellulosic Transportation Fuels. Angew. Chem. Int. Ed. 2010, 49, 4479–4483.
- Chen, J.; Zhang, W.; Tan, L.; Wang, Y.; He, G. Optimization of metabolic pathways for bioconversion of lignocellulose to ethanol through genetic engineering. Biotechnol. Adv. 2009, 27, 593–598.
- Li, J.; Liu, J.; Liu, H.; Xu, G.; Zhang, J.; Liu, J.; Zhou, G.; Li, Q.; Xu, Z.; Fu, Y. Selective Hydrodeoxygenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran over Heterogeneous Iron Catalysts. ChemSusChem 2017, 10, 1436–1447.
- Ayed, C.; Huang, W.; Kizilsavas, G.; Landfester, K.; Zhang, K.A.I. Photocatalytic Partial Oxidation of 5-Hydroxymethylfurfural (HMF) to 2,5-Diformylfuran (DFF) Over a Covalent Triazine Framework in Water. ChemPhotoChem 2020, 4, 571–576.
- Cai, J.; Li, K.; Wu, S. Recent advances in catalytic conversion of biomass derived 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid. Biomass Bioenergy 2022, 158, 106358.
- Eerhart, A.J.J.E.; Faaij, A.P.C.; Patel, M.K. Replacing fossil based PET with biobased PEF; process analysis, energy and GHG balance. Energy Environ. Sci. 2012, 5, 6407.
- Geng, Y.; Wang, Z.; Hu, X.; Li, Y.; Zhang, Q.; Li, Y.; Wang, R.; Zhang, L. Bio-based polyesters based on 2,5-furandicarboxylic acid as 3D-printing materials: Design, preparation and performances. Eur. Polym. J. 2019, 114, 476–484.
- Sun, Y.; Liu, P.; Liu, Z. Catalytic conversion of carbohydrates to 5-hydroxymethylfurfural from the waste liquid of acid hydrolysis NCC. Carbohydr. Polym. 2016, 142, 177–182.
- Chidambaram, M.; Bell, A.T. A two-step approach for the catalytic conversion of glucose to 2,5-dimethylfuran in ionic liquids. Green Chem. 2010, 12, 1253.
- Zhou, X.; Zhang, Z.; Liu, B.; Xu, Z.; Deng, K. Microwave-assisted rapid conversion of carbohydrates into 5-hydroxymethylfurfural by ScCl3 in ionic liquids. Carbohydr. Res. 2013, 375, 68–72.
- Hou, Q.; Zhen, M.; Li, W.; Liu, L.; Liu, J.; Zhang, S.; Nie, Y.; Bai, C.; Bai, X.; Ju, M. Efficient catalytic conversion of glucose into 5-hydroxymethylfurfural by aluminum oxide in ionic liquid. Appl. Catal. B Environ. 2019, 253, 1–10.
- Pang, J.; Sun, J.; Zheng, M.; Li, H.; Wang, Y.; Zhang, T. Transition metal carbide catalysts for biomass conversion: A review. Appl. Catal. B Environ. 2019, 254, 510–522.
- Zhong, R.; Sels, B.F. Sulfonated mesoporous carbon and silica-carbon nanocomposites for biomass conversion. Appl. Catal. B Environ. 2018, 236, 518–545.
- Han, X.; Li, C.; Liu, X.; Xia, Q.; Wang, Y. Selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over MnOx–CeO2 composite catalysts. Green Chem. 2017, 19, 996–1004.
- Nguyen, C.V.; Liao, Y.-T.; Kang, T.-C.; Chen, J.E.; Yoshikawa, T.; Nakasaka, Y.; Masuda, T.; Wu, K.C.-W. A metal-free, high nitrogen-doped nanoporous graphitic carbon catalyst for an effective aerobic HMF-to-FDCA conversion. Green Chem. 2016, 18, 5957–5961.
- Li, X.; Sun, M.; Rooke, J.C.; Chen, L.; Su, B.-L. Synthesis and applications of hierarchically porous catalysts. Chin. J. Catal. 2013, 34, 22–47.
- Ding, M.; Liu, X.; Ma, P.; Yao, J. Porous materials for capture and catalytic conversion of CO2 at low concentration. Coord. Chem. Rev. 2022, 465, 214576.
- Sun, M.-H.; Huang, S.-Z.; Chen, L.-H.; Li, Y.; Yang, X.-Y.; Yuan, Z.-Y.; Su, B.-L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563.
- Zhao, X.S.; Bao, X.Y.; Guo, W.; Lee, F.Y. Immobilizing catalysts on porous materials. Mater. Today 2006, 9, 32–39.
- Gupta, D.; Kumar, R.; Pant, K.K. Hydrotalcite supported bimetallic (Ni-Cu) catalyst: A smart choice for one-pot conversion of biomass-derived platform chemicals to hydrogenated biofuels. Fuel 2020, 277, 118111.
- Xu, J.; Wang, L. Carbon Nanomaterials//Nano-Inspired Biosensors for Protein Assay with Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–38.
- Zhao, S.; Wang, D.; Amal, R.; Dai, L. Carbon-Based Metal-Free Catalysts for Key Reactions Involved in Energy Conversion and Storage. Adv. Mater. 2019, 31, 1801526.
- Giannakoudakis, D.A.; Zormpa, F.F.; Margellou, A.G.; Qayyum, A.; Len, C.; Colmenares, J.C.; Triantafyllidis, K.S. Carbon-Based Nanocatalysts (CnCs) for Biomass Valorization and Hazardous Organics Remediation. Nanomaterials 2022, 12, 1679.
- Pikunic, J.; Clinard, C.; Cohaut, N.; Gubbins, K.E.; Guet, J.-M.; Pellenq, R.J.-M.; Rannou, I.; Rouzaud, J.-N. Structural Modeling of Porous Carbons: Constrained Reverse Monte Carlo Method. Langmuir 2003, 19, 8565–8582.
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, Covalent Triazine-Based Frameworks Prepared by Ionothermal Synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453.
- Xu, Y.; Mao, N.; Feng, S.; Zhang, C.; Wang, F.; Chen, Y.; Zeng, J.; Jiang, J.-X. Perylene-Containing Conjugated Microporous Polymers for Photocatalytic Hydrogen Evolution. Macromol. Chem. Phys. 2017, 218, 1700049.
- Sharma, V.; Sahoo, A.; Sharma, Y.; Mohanty, P. Synthesis of nanoporous hypercrosslinked polyaniline (HCPANI) for gas sorption and electrochemical supercapacitor applications. RSC Adv. 2015, 5, 45749–45754.
- Ding, X.; Han, B.-H. Metallophthalocyanine-Based Conjugated Microporous Polymers as Highly Efficient Photosensitizers for Singlet Oxygen Generation. Angew. Chem. Int. Ed. 2015, 54, 6536–6539.
- Totten, R.K.; Olenick, L.L.; Kim, Y.-S.; Chakraborty, S.; Weston, M.H.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. A dual approach to tuning the porosity of porous organic polymers: Controlling the porogen size and supercritical CO2 processing. Chem. Sci. 2014, 5, 782–787.
- Lu, S.; Liu, Q.; Han, R.; Guo, M.; Shi, J.; Song, C.; Ji, N.; Lu, X.; Ma, D. Potential applications of porous organic polymers as adsorbent for the adsorption of volatile organic compounds. J. Environ. Sci. 2021, 105, 184–203.
- Li, Z.; Yang, Y. Macrocycle-Based Porous Organic Polymers for Separation, Sensing, and Catalysis. Adv. Mater. 2022, 34, 2107401.
- Luo, D.; Li, M.; Ma, Q.; Wen, G.; Dou, H.; Ren, B.; Liu, Y.; Wang, X.; Shui, L.; Chen, Z. Porous organic polymers for Li-chemistry-based batteries: Functionalities and characterization studies. Chem. Soc. Rev. 2022, 51, 2917–2938.
- Liu, X.; Liu, C.; Lai, W.; Lai, W. Porous Organic Polymers as Promising Electrode Materials for Energy Storage Devices. Adv. Mater. Technol. 2020, 5, 2000154.
- Tang, Y.; Varyambath, A.; Ding, Y.; Chen, B.; Huang, X.; Zhang, Y.; Yu, D.; Kim, I.; Song, W. Porous organic polymers for drug delivery: Hierarchical pore structures, variable morphologies, and biological properties. Biomater. Sci. 2022, 10, 5369–5390.
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’keeffe, M.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170.
- Liu, J.T.; Wang, N.; Ma, L. Recent Advances in Covalent Organic Frameworks for Catalysis. Chem. Asian J. 2020, 15, 338–351.
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmins, J.M.; et al. Targeted Synthesis of a Porous Aromatic Framework with High Stability and Exceptionally High Surface Area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460.
- Díaz, U.; Corma, A. Ordered covalent organic frameworks, COFs and PAFs. From preparation to application. Coord. Chem. Rev. 2016, 311, 85–124.
- Yuan, R.; Ren, H.; Yan, Z.; Wang, A.; Zhu, G. Robust tri(4-ethynylphenyl)amine-based porous aromatic frameworks for carbon dioxide capture. Polym. Chem. 2014, 5, 2266.
- Wood, C.D.; Tan, B.; Trewin, A.; Niu, H.; Bradshaw, D. Hydrogen Storage in Microporous Hypercrosslinked Organic Polymer Networks. Chem. Mater. 2007, 19, 2034–2048.
- Gao, H.; Ding, L.; Li, W.; Ma, G.; Bai, H.; Li, L. Hyper-Cross-Linked Organic Microporous Polymers Based on Alternating Copolymerization of Bismaleimide. ACS Macro Lett. 2016, 5, 377–381.
- Msayib, K.J.; McKeown, N.B. Inexpensive polyphenylene network polymers with enhanced microporosity. J. Mater. Chem. A 2016, 4, 10110–10113.
- Li, B.; Gong, R.; Wang, W.; Huang, X.; Zhang, W.; Li, H.; Hu, C.; Tan, B. A New Strategy to Microporous Polymers: Knitting Rigid Aromatic Building Blocks by External Cross-Linker. Macromolecules 2011, 44, 2410–2414.
- Tan, Z.; Chen, K.; Liu, P. Possibilities and challenges of China's forestry biomass resource utilization. Renew. Sustain. Energy Rev. 2015, 41, 368–378.
- Peng, W.-H.; Lee, Y.-Y.; Wu, C.; Wu, K.C.-W. Acid–base bi-functionalized, large-pored mesoporous silica nanoparticles for cooperative catalysis of one-pot cellulose-to-HMF conversion. J. Mater. Chem. 2012, 22, 23181.
- Binder, J.B.; Cefali, A.V.; Blank, J.J.; Raines, R.T. Mechanistic insights on the conversion of sugars into 5-hydroxymethylfurfural. Energy Environ. Sci. 2010, 3, 765.
- Moreau, C.; Durand, R.; Razigade, S.; Duhamet, J.; Faugeras, P. Dehydration of fructose to 5-hydroxymethylfurfural over H-mordenites. Appl. Catal. A Gen. 1996, 145, 211–224.
- Wang, T.; Nolte, M.W.; Shanks, B.H. Catalytic dehydration of C6 carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 2014, 16, 548–572.
- Li, C.; Zhang, Z.; Zhao, Z.K. Direct conversion of glucose and cellulose to 5-hydroxymethylfurfural in ionic liquid under microwave irradiation. Tetrahedron Lett. 2009, 50, 5403–5405.
- Binder, J.B.; Raines, R.T. Simple Chemical Transformation of Lignocellulosic Biomass into Furans for Fuels and Chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985.
- He, M.; Sun, Y.; Han, B. Green Carbon Science: Efficient Carbon Resource Processing, Utilization, and Recycling towards Carbon Neutrality. Angew. Chem. Int. Ed. 2022, 61, e202112835.
- Enjamuri, N.; Sarkar, S.; Reddy, B.M.; Mindal, J. Design and Catalytic Application of Functional Porous Organic Polymers: Opportunities and Challenges. Chem. Rec. 2019, 19, 1782–1792.
- Haase, F.; Lotsch, B.V. Solving the COF trilemma: Towards crystalline, stable and functional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 8469–8500.
- Peng, Y.; Hu, Z.; Gao, Y.; Yuan, D.; Kang, Z.; Qian, Y.; Yan, N.; Zhao, D. Synthesis of a Sulfonated Two-Dimensional Covalent Organic Framework as an Efficient Solid Acid Catalyst for Biobased Chemical Conversion. ChemSusChem 2015, 8, 3208–3212.
- Chen, D.; Chen, W.; Xing, G.; Zhang, T.; Chen, L. An Upgraded “Two-in-One” Strategy toward Highly Crystalline Covalent Organic Frame-works. Chem. A Eur. J. 2020, 26, 8377–8381.
- Desir, P.; Saha, B.; Vlachos, D.G. Ultrafast flow chemistry for the acid-catalyzed conversion of fructose. Energy Env. Ment. Sci. 2019, 12, 2463–2475.
- Du, M.; Agrawal, A.M.; Chakraborty, S.; Garibay, S.J.; Limvorapitux, R.; Choi, B.; Madrahimov, S.T.; Nguyen, S.T. Matching the Activity of Homogeneous Sulfonic Acids: The Fructose-to-HMF Conversion Catalyzed by Hierarchically Porous Sulfonic-Acid-Functionalized Porous Organic Polymer (POP) Catalysts. ACS Sustain. Chem. Eng. 2019, 7, 8126–8135.
- Das, S.K.; Chatterjee, S.; Mondal, S.; Bhaumik, A. A new triazine-thiophene based porous organic polymer as efficient catalyst for the synthesis of chromenes via multicomponent coupling and catalyst support for facile synthesis of HMF from carbohydrates. Mol. Catal. 2019, 475, 110483.
- Dong, K.; Zhang, J.; Luo, W.; Su, L.; Huang, Z. Catalytic conversion of carbohydrates into 5-hydroxymethyl furfural over sulfonated hyper-cross-linked polymer in DMSO. Chem. Eng. J. 2018, 334, 1055–1064.
- Testa, M.L.; La Parola, V. Sulfonic Acid-Functionalized Inorganic Materials as Efficient Catalysts in Various Applications: A Minireview. Catalysts 2021, 11, 1143.
- Ravi, S.; Choi, Y.; Choe, J.K. Achieving effective fructose-to-5-hydroxymethylfurfural conversion via facile synthesis of large surface phosphate-functionalized porous organic polymers. Appl. Catal. B Environ. 2020, 271, 118942.
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963.
- De Jong, E.; Dam, M.A.; Sipos, L.; Gruter, G.-J.M. Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters; Smith, P.B., Gross, R.A., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012; Volume 115, pp. 1–13.
- Zhang, W.; Qian, H.; Hou, Q.; Ju, M. The functional and synergetic optimization of the thermal-catalytic system for the selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran: A review. Green Chem. 2023, 25, 893–914.
- Li, C.; Na, Y. Recent Advances in Photocatalytic Oxidation of 5-Hydroxymethylfurfural. ChemPhotoChem 2021, 5, 502–511.
- Yang, Y.; Mu, T. Electrochemical oxidation of biomass derived 5-hydroxymethylfurfural (HMF): Pathway, mechanism, catalysts and coupling reactions. Green Chem. 2021, 23, 4228–4254.
- Cunha, J.T.; Romaní, A.; Domingues, L. Whole Cell Biocatalysis of 5-Hydroxymethylfurfural for Sustainable Biorefineries. Catalysts 2022, 12, 202.
- Yang, D.-H.; Tao, Y.; Ding, X.; Han, B.-H. Porous organic polymers for electrocatalysis. Chem. Soc. Rev. 2022, 51, 761–791.
- Cai, M.; Ding, S.; Gibbons, B.; Yang, X.; Kessinger, M.C.; Morris, A.J. Nickel(II)-modified covalent-organic framework film for electrocatalytic oxidation of 5-hydroxymethylfurfural (HMF). Chem. Commun. 2020, 56, 14361–14364.
- Pal, P.; Saravanamurugan, S. Recent Advances in the Development of 5-Hydroxymethylfurfural Oxidation with Base (Nonpre-cious)-Metal-Containing Catalysts. ChemSusChem 2019, 12, 145–163.
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306.
- Brandolese, A.; Ragno, D.; Di Carmine, G.; Bernardi, T.; Bortolini, O.; Paolo Giovannini, P.; Ginoble Pandoli, O.; Altomare, A.; Massi, A. Aerobic oxidation of 5-hydroxymethylfurfural to 5-hydroxymethyl-2-furancarboxylic acid and its derivatives by heterogeneous NHC-catalysis. Org. Biomol. Chem. 2018, 16, 8955–8964.
- Galkin, K.I.; Ananikov, V.P. When Will 5-Hydroxymethylfurfural, the “Sleeping Giant” of Sustainable Chemistry, Awaken? ChemSusChem 2019, 12, 2976–2982.
- Chen, J.; Liu, R.; Guo, Y.; Chen, L.; Gao, H. Selective Hydrogenation of Biomass-Based 5-Hydroxymethylfurfural over Catalyst of Palladium Immobilized on Amine-Functionalized Metal–Organic Frameworks. ACS Catalysis 2015, 5, 722–733.
- Yao, Y.; Wu, X.; Gutiérrez, O.Y.; Ji, J.; Wang, S.; Xu, Y.; Zhao, Y.; Wang, S.; Ma, X.; Lercher, J.A. Roles of Cu+ and Cu0 sites in liquid-phase hydrogenation of esters on core-shell catalysts. Appl. Catal. B Environ. 2020, 267, 118698.
- Yue, H.; Zhao, Y.; Zhao, S.; Wang, B.; Ma, X.; Gong, J. A copper-phyllosilicate core-sheath nanoreactor for carbon–oxygen hydrogenolysis reactions. Nat. Commun. 2013, 4, 2339.
- Kong, X.; Zhu, Y.; Fang, Z.; Kozinski, J.A.; Butter, L.S.; Xu, L.; Song, H.; Wei, X. Catalytic conversion of 5-hydroxymethylfurfural to some value-added derivatives. Green Chem. 2018, 20, 3657–3682.
- Wang, J.; Zhang, Z.; Jin, S.; Shen, X. Efficient conversion of carbohydrates into 5-hydroxylmethylfurfan and 5-ethoxymethylfurfural over sufonic acid-functionalized mesoporous carbon catalyst. Fuel 2017, 192, 102–107.
- Zhang, J.; Dong, K.; Luo, W.; Guan, H. Catalytic upgrading of carbohydrates into 5-ethoxymethylfurfural using SO3H functionalized hyper-cross-linked polymer based carbonaceous materials. Fuel 2018, 234, 664–673.
- Lai, J.; Cheng, F.; Zhou, S.; Wen, S.; Guo, D.; Zhao, W.; Liu, X.; Yin, D. Base-free oxidation of 5-hydroxymethylfurfural to 2, 5-furan dicarboxylic acid over nitro-gen-containing polymers supported Cu-doped MnO2 nanowires. Appl. Surf. Sci. 2021, 565, 150479.
- Modak, A.; Nandi, M.; Mondal, J.; Bhaumik, A. Porphyrin based porous organic polymers: Novel synthetic strategy and exceptionally high CO2 adsorption capacity. Chem. Commun. 2012, 48, 248–250.
- Saha, B.; Gupta, D.; Abu-Omar, M.M.; Modak, A.; Bhaumik, A. Porphyrin-based porous organic polymer-supported iron(III) catalyst for efficient aerobic oxidation of 5-hydroxymethyl-furfural into 2,5-furandicarboxylic acid. J. Catal. 2013, 299, 316–320.
This entry is offline, you can click here to edit this entry!
