The “extra-situ” and “in situ” sol-gel routes to the synthesis of silica/epoxy nanocomposites are discussed. Based on the reported results, the “in situ” strategy is expected to be applicable to produce nanocomposites of different composition for both filler than matrix nature and with dispersed phases more complex than the one already obtained till today with this simple and eco-friendly strategy
- sol–gel
- silica in situ formation mechanism
- highly engineered nanoparticles
- nanocomposites
- epoxy
- flame retardancy
1. Introduction
The development of organic/inorganic hybrid materials received very great attention for their widespread applications in the fields of catalysis, biomaterials, electrochemistry, energy storage devices, etc. The dispersed phase may have size so small as a few nanometers. In particular silica/epoxy system was recently actively studied. In fact, silica is one of the most studied fillers added to improve electrical insulation, anticorrosion and mechanical (elastic modulus, hardness, impact re-sistance, and fracture toughness) properties of epoxies.
The sol-gel chemistry proves to be a valuable route for the synthesis of hybrid materials, particularly highly engineered silica nanoparticles and silica/epoxy nanocomposites with enhanced properties with respect to neat epoxy matrix [1-4]. The topic was recently revised by the authors [5].
2. The extra situ sol-gel route
Well assessed sol-gel procedures allow to prepare monodisperse silica nanoparticles of easily tunable size in the range from few nm to 1μm [6-7]. The mild synthesis conditions allow also to produ ce, with the help of surfactant molecules, mesoporous nanoparticles possessing high specific surface as high as 1000 m2/g [8-12] also in the form of wrinkled particles [13]. The nanoparticles surface may be easily modified [14-15] through the use of silane coupling agent (see scheme 1) in order to well disperse them in the polymeric matrix or even tailor the interface as a function of the applications. Moreover, they may be the core of highly sophisticated and complex nanoparticles specifically designed for the application of interest. There are a lot of strategies well described in the literature many of them exploiting the basic sol-gel reaction scheme [16]. A list, of course not exhaustive, of nanocomposites obtained by dispersion of these nanoparticles in epoxy (extra situ procedure) has been given in the above reminded review [5, 17-21].
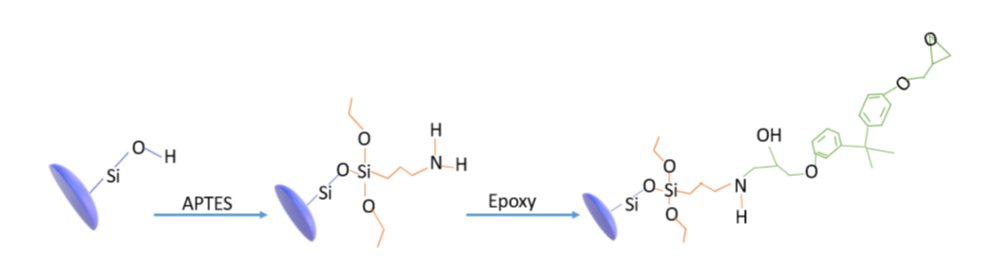
Scheme 1. Functionalization through coupling agent. APTES anchors to the silica surface thanks to condensation reaction with silicatic surface silanols. The reaction of amino group with oxirane of epoxy allows, finally, a strong covalent bond matrix/silcatic surface to be set up.
3. The “in situ” sol-gel route
A particular focus was put, in the review, on the synthesis of nanocomposites with “in situ” generation of silica nanoparticles exploiting the mildness of sol-gel synthesis conditions allowing to prepare the nanoparticles in the presence also of monomers or polymers. The “extra situ” route, in fact, allows to obtain interesting complex functional nanomaterials at the expense of the use of large quantities of solvents, which often are very harmful. Moreover, the synthesis requires several successive processing steps for the nanoparticles synthesis, their functionalization, and multiple washing and solvent removal operations. “In situ” processes may be designed in which the involved solvent is significantly reduced while the time and money consuming steps of the “extra situ” method are overcome. Many authors appropriately speak, in this case, of “Solvent-free One-Pot” processes. The “in situ” methodology may be brought back to four processes [22], differing for the order of addition of the reagents (epoxy resin, curing agent, the inorganic precursor, usually a metal alkoxide including TEOS, water, alcohol and a coupling agent) in one only pot, so as illustrated in scheme 2.
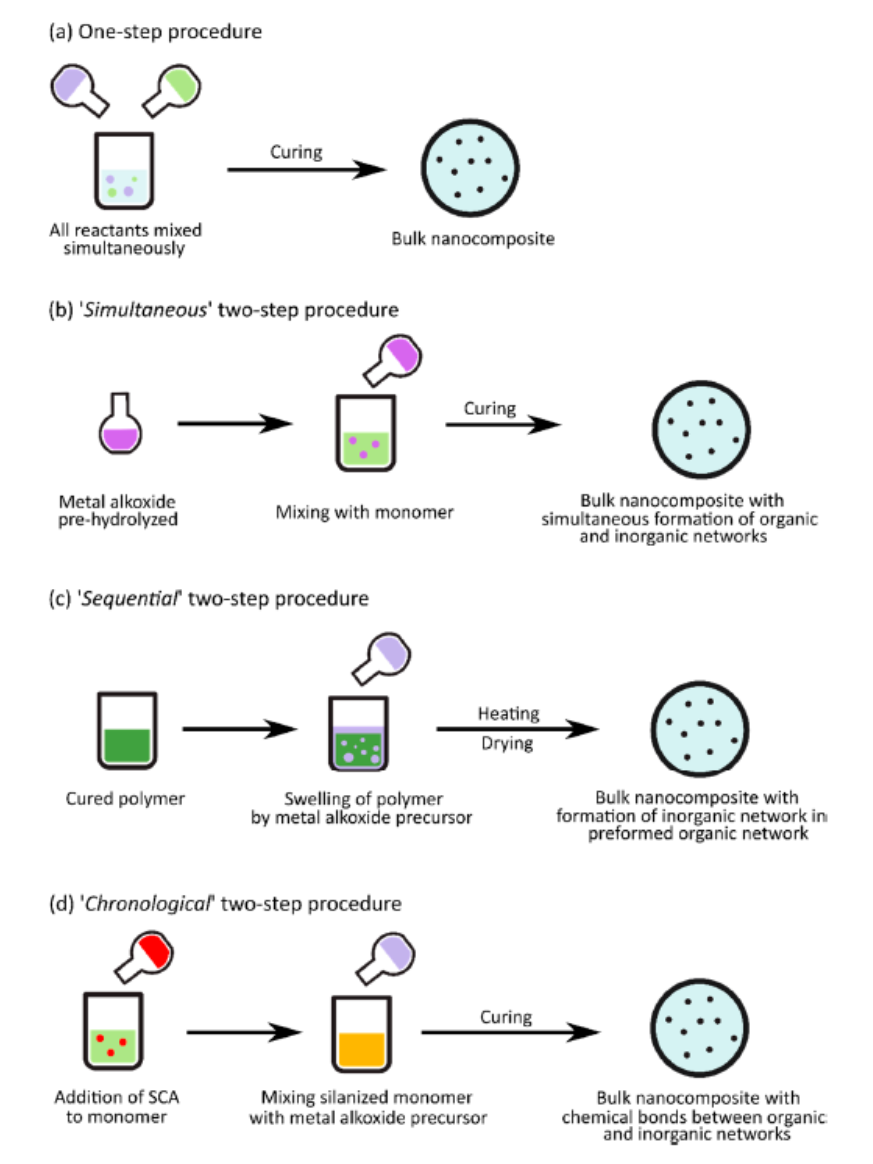
Scheme 2. Scheme of (a) one-step procedure, (b) simultaneous, (c) sequential, and (d) chronological two-step procedure. The difference is in the order of addition of reagents. Reprinted with permission from [22]. Copyright 2023 MDPI
Different coupling agent exposing amino (APTMS and APTES), glycidoxy (GPTMS) or isocyanato (IPTS) functional groups were largely used [5]. Improvements of thermal stability, mechanical properties, glass transition temperature, storage modulus, barrier properties and better high voltage insulation with respect to the neat epoxy ones were reported [5].
More recently relevant modifications to the procedures were applied. The first one is relative to the use of ionic liquids, in conjunction or alternative to silane coupling agent, [23-25]. It’s worth reminding that they have been already used in the literature as template in the synthesis of mesoporous nanoparticles. The authors obtained rubbery nanocomposites possessing till 6 times higher modulus and tensile strength as well as more than 10 times higher energy to break than unmodified silica/epoxy. The beneficial effects of the nanosized silica obtained through the “in situ” route on the fire behaviour were described in several papers [26-30]. Very recently it was proved that, even at content as low as 2%, the “in situ” nanosilica strongly improved the fire behavior [26]. It was also demonstrated that it was possible to easily introduce flame retardants in order to reach a UL 94-V0 rating corresponding to self-extinguishing fire behaviour [27-28]. The “in situ” nanosilica played a synergistic role with traditional phosphorous based flame retardants allowing to reach the result with very low P contents. Also, humic acid, a known biomass, could be introduced as flame retardant assuring, in formulations with other traditional ones, UL94-V0 rating, as a good example of waste-to-wealth transformation [31]. On one hand this opens the application perspective. In fact, very often the satisfaction of severe fire safety regulations is a “sine qua non” prerequisite for the use. On the other hand, this proves that the “in situ” strategies may go beyond the traditional reagents batch compositions comprising strictly polymeric and nanosilica precursors.
4. Insights into the ‘In Situ’ nanocomposite structure and formation through HRTEM and SAXS
Finally, very recently the use of HRTEM and combined small and wide-angle X-ray scattering (WAXS-SAXS) by means of a multirange Ganesha 300 XL+ device over an unprecedented q range (0.02–25 nm−1) proved that the ‘in situ’ nanosilica has a surprising ordered structure [32-33] according also to reported NMR results that do not agree with the gel structure expected for the Stober particles. In fact, it was proven that, at least in the course of the “chronological two step” procedure, multisheet silica nanoparticles form. A mechanism, borrowed from the classical theory of crystallization of inorganic glasses, has been proposed [32-33] that allows to give an explanation also to other NMR, FTIR, DMA results collected on the same system. A new interpretation of the origin of the bi-continuous phases structure reported by many authors was given [33]. It’s worth pointing out that the mechanism foresees the possibility to synthesize nanocomposites of different both matrix and filler nature and defines the characteristics of the precursor [34]. The synthesis of a nanocomposite containing Mg(OH)2 nanocrystals seems to support these expectations [34]. The “in situ” strategy appears, therefore, to be a very promising synthesis strategy to produce in an easy and eco-friendly manner nanocomposites (not only epoxy/silica) of outstanding applications.
5. Conclusions
In conclusion, the extra situ strategy allows us to produce very complex nanoparticles to disperse into polymeric matrices. The in situ one is, instead, a very promising synthesis strategy that allows us to produce nanocomposites in an easier and eco-friendly manner (not only epoxy/silica systems) for outstanding applications. The proposed mechanism of nanoparticles formation could also help to design synthesis strategies of nanocomposites with in situ-generated nanoparticles more complex than the ones obtained, until today, with this strategy.
References
1) A. Serra, X. Ramis, and X. Fernández-Francos, “Epoxy sol-gel hybrid thermosets,” Coatings, vol. 6, no. 1. MDPI AG, Mar. 01, 2016. doi: 10.3390/coatings6010008.
2) S. Brinker, In Sol-gel science: the physics and chemistry of sol-gel processing. 1990.
3) B. L. Cushing, V. L. Kolesnichenko, and C. J. O’Connor, “Recent advances in the liquid-phase syntheses of inorganic nanoparticles,” Chem Rev, vol. 104, no. 9, pp. 3893–3946, Sep. 2004, doi: 10.1021/cr030027b.
4) F. Branda, “The Sol-Gel Route to Nanocomposites.” [Online]. Available: www.intechopen.com
5) F.Branda, R.Grappa, A.Costantini and G.Luciani, “Sol–gel approach for fabricating silica/epoxy nNanocomposites” Polymers 2023, 15, 2987. https://doi.org/10.3390/polym15142987
6) W. Stober, A. Fink, and D. Ernst Bohn, “Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range 1,” 1968.
7) G. H. Bogush, M. A. Tracy, and C. F. Zukoski Iv, “PREPARATION OF MONODISPERSE SILICA PARTICLES: CONTROL OF SIZE AND MASS FRACTION,” 1988.
8) F. Di Renzo et al., “MICROPOROUS MATERIALS A 28-year-old synthesis of micelle-templated mesoporous silica,” 1997.
9) A. Katiyar, S. Yadav, P. G. Smirniotis, and N. G. Pinto, “Synthesis of ordered large pore SBA-15 spherical particles for adsorption of biomolecules,” J Chromatogr A, vol. 1122, no. 1–2, pp. 13–20, Jul. 2006, doi: 10.1016/j.chroma.2006.04.055.
10) Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science. 1998;279:548–52
11) D. Zhao, Q. Huo, J. Feng, B. F. Chmelka, and G. D. Stucky, “Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures,” 1998. [Online]. Available: https://pubs.acs.org/sharingguidelines
12) F.Branda “Synthesis and Functionalization of Mesoporous Bioactive Glasses for Drug Delivery” in Clinical Applications of Biomaterials – State of the art Progress Trends and Novel ApproachesG.Kaur editor, Springer International Publishing AG 2017, ISBN 978-3-319-56058-8, ISBN 978-3-319-56059-5 (eBook) DOI 10.1007/978-3-319-56059-5
13) D. S. Moon and J. K. Lee, “Tunable synthesis of hierarchical mesoporous silica nanoparticles with radial wrinkle structure,” Langmuir, vol. 28, no. 33, pp. 12341–12347, Aug. 2012, doi: 10.1021/la302145j
14) F. Ahangaran and A. H. Navarchian, “Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: A review,” Advances in Colloid and Interface Science, vol. 286. Elsevier B.V., Dec. 01, 2020. doi: 10.1016/j.cis.2020.102298.
15) T. Aziz et al., “Recent Progress in Silane Coupling Agent with Its Emerging Applications,” Journal of Polymers and the Environment, vol. 29, no. 11. Springer, pp. 3427–3443, Nov. 01, 2021. doi: 10.1007/s10924-021-02142-1.
16) E. Bourgeat-Lami, “Hybrid Organic/Inorganic Particles,” in Hybrid Materials: Synthesis, Characterization, and Applications, John Wiley and Sons, 2007, pp. 87–149. doi: 10.1002/9783527610495.ch3.
17) S. Ghiyasi et al., “Hyperbranched poly(ethyleneimine) physically attached to silica nanoparticles to facilitate curing of epoxy nanocomposite coatings,” Prog Org Coat, vol. 120, pp. 100–109, Jul. 2018, doi: 10.1016/j.porgcoat.2018.03.019.
18) M. Jouyandeh, O. M. Jazani, A. H. Navarchian, M. Shabanian, H. Vahabi, and M. R. Saeb, “Surface engineering of nanoparticles with macromolecules for epoxy curing: Development of super-reactive nitrogen-rich nanosilica through surface chemistry manipulation,” Appl Surf Sci, vol. 447, pp. 152–164, Jul. 2018, doi: 10.1016/j.apsusc.2018.03.197.
19) S. A. Haddadi et al., “Zinc-doped silica/polyaniline core/shell nanoparticles towards corrosion protection epoxy nanocomposite coatings,” Compos B Eng, vol. 212, May 2021, doi: 10.1016/j.compositesb.2021.108713.
20) Y. Q. Li, S. Y. Fu, Y. Yang, and Y. W. Mai, “Facile synthesis of highly transparent polymer nanocomposites by introduction of core-shell structured nanoparticles,” Chemistry of Materials, vol. 20, no. 8, pp. 2637–2643, Apr. 2008, doi: 10.1021/cm7033307.
21) C. R. Picu et al., “Toughening in nanosilica-reinforced epoxy with tunable filler-matrix interface properties,” Compos Sci Technol, vol. 183, Oct. 2019, doi: 10.1016/j.compscitech.2019.107799.
22) M. M. Adnan, A. R. M. Dalod, M. H. Balci, J. Glaum, and M. A. Einarsrud, “In situ synthesis of hybrid inorganic-polymer nanocomposites,” Polymers, vol. 10, no. 10. MDPI AG, Oct. 11, 2018. doi: 10.3390/polym10101129
23) R. K. Donato et al., “The multifunctional role of ionic liquids in the formation of epoxy-silica nanocomposites,” J Mater Chem, vol. 21, no. 36, pp. 13801–13810, Sep. 2011, doi: 10.1039/c1jm11752a.
24) R. K. Donato, K. Z. Donato, H. S. Schrekker, and L. Matějka, “Tunable reinforcement of epoxy-silica nanocomposites with ionic liquids,” J Mater Chem, vol. 22, no. 19, pp. 9939–9948, May 2012, doi: 10.1039/c2jm30830d.
25) R. K. Donato et al., “Epoxy-silica nanocomposite interphase control using task-specific ionic liquids via hydrolytic and non-hydrolytic sol-gel processes,” RSC Adv, vol. 5, no. 111, pp. 91330–91339, Oct. 2015, doi: 10.1039/c5ra18387a.
26) A. Bifulco et al., “Effects of post cure treatment in the glass transformation range on the structure and fire behavior of in situ generated silica/epoxy hybrids,” J Solgel Sci Technol, vol. 87, no. 1, pp. 156–169, Jul. 2018, doi: 10.1007/s10971-018-4710-2.
27) A. Bifulco et al., “Fire and mechanical properties of DGEBA-based epoxy resin cured with a cycloaliphatic hardener: Combined action of silica, melamine and DOPO-derivative,” Mater Des, vol. 193, Aug. 2020, doi: 10.1016/j.matdes.2020.108862.
28) A. Bifulco et al., “Improving flame retardancy of in-situ silica-epoxy nanocomposites cured with aliphatic hardener: Combined effect of DOPO-based flame-retardant and melamine,” Composites Part C: Open Access, vol. 2, Oct. 2020, doi: 10.1016/j.jcomc.2020.100022.
29) A. Bifulco et al., “Thermal and fire behavior of a bio-based epoxy/silica hybrid cured with methyl nadic anhydride,” Polymers (Basel), vol. 12, no. 8, Aug. 2020, doi: 10.3390/POLYM12081661.
30) A. Bifulco et al., “In Situ P-Modified Hybrid Silica-Epoxy Nanocomposites via a Green Hydrolytic Sol-Gel Route for Flame-Retardant Applications,” ACS Appl Nano Mater, 2023, doi: 10.1021/acsanm.3c00590.
31) V. Venezia et al., “Detailed Thermal, Fire, and Mechanical Study of Silicon-Modified Epoxy Resin Containing Humic Acid and Other Additives,” ACS Appl Polym Mater, vol. 3, no. 11, pp. 5969–5981, Nov. 2021, doi: 10.1021/acsapm.1c01240.
32) F. Branda et al., “Structure and Bottom-up Formation Mechanism of Multisheet Silica-Based Nanoparticles Formed in an Epoxy Matrix through an In-Situ Process,” Langmuir, vol. 37, no. 29, pp. 8886–8893, Jul. 2021, doi: 10.1021/acs.langmuir.1c01363.
33) F. Branda et al., “Effect of the Coupling Agent (3-Aminopropyl) Triethoxysilane on the Structure and Fire Behavior of Solvent-Free One-Pot Synthesized Silica-Epoxy Nanocomposites,” Polymers (Basel), vol. 14, no. 18, Sep. 2022, doi: 10.3390/polym14183853.
34) F. Branda, J. Passaro, R. Pauer, S. Gaan, and A. Bifulco, “Solvent-Free One-Pot Synthesis of Epoxy Nanocomposites Containing Mg(OH)2Nanocrystal-Nanoparticle Formation Mechanism,” Langmuir, 2022, doi: 10.1021/acs.langmuir.2c00377.
This entry is adapted from the peer-reviewed paper 10.3390/polym15142987
