With the advantages offered by cationic photopolymerization (CP) such as broad wavelength activation, tolerance to oxygen, low shrinkage and the possibility of “dark cure”, it has attracted extensive attention in photoresist, deep curing and other fields in recent years. The applied photoinitiating systems (PIS) play a crucial role as they can affect the speed and type of the polymerization and properties of the materials formed. Much effort has been invested into developing cationic photoinitiating systems (CPISs) that can be activated at long wavelengths and overcome technical problems and challenges faced.
- photopolymerization
- cationic photoinitiating systems
- onium salt
- UV/visible LED lights
- long wavelength
1. Introduction
2. Ionic Photoinitiators
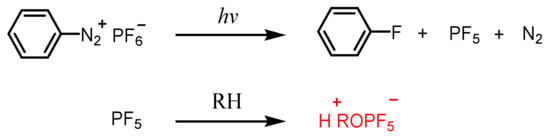
2.1. Aryl Diazonium Salts
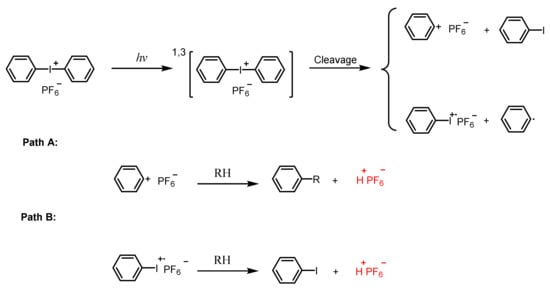
2.2. Iodonium Salts
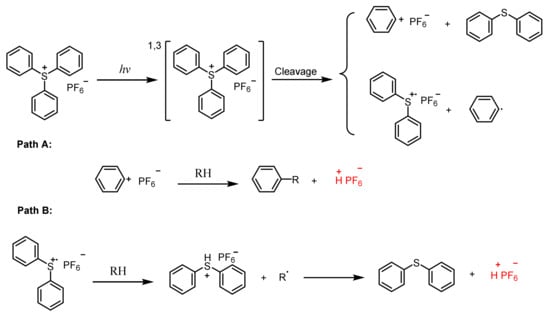
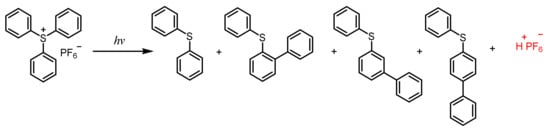
2.3. Sulfonium Salts
Triarylsulfonium Salts
Aryl-Alkylsulfonium Salts
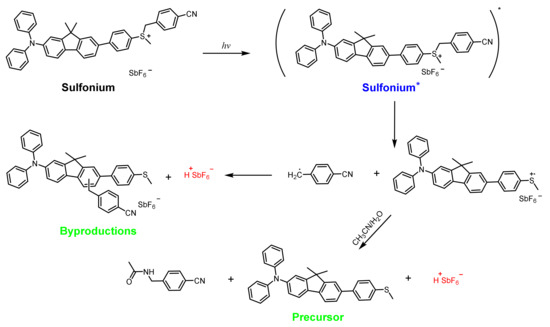
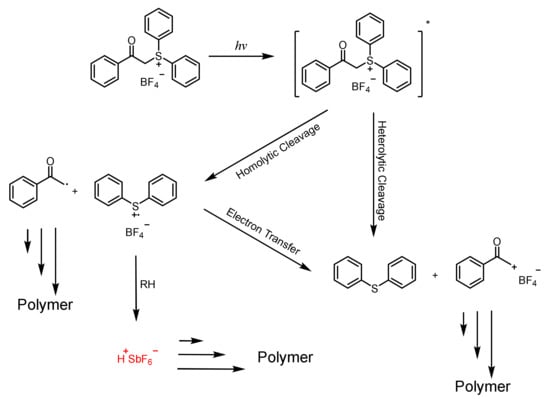
Phenacylsulfonium Salts
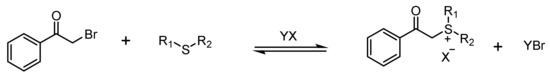
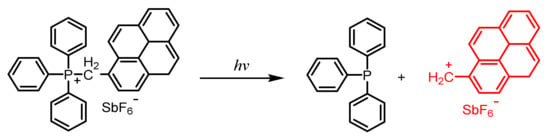
2.4. Phosphonium Salts
2.5. Ammonium Salts
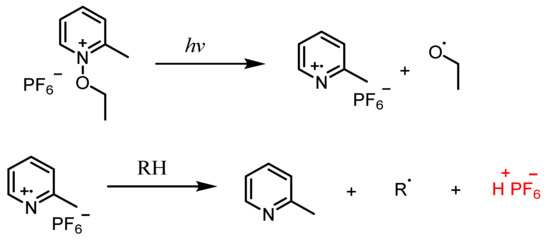
N-Alkoxypyridinium Salts
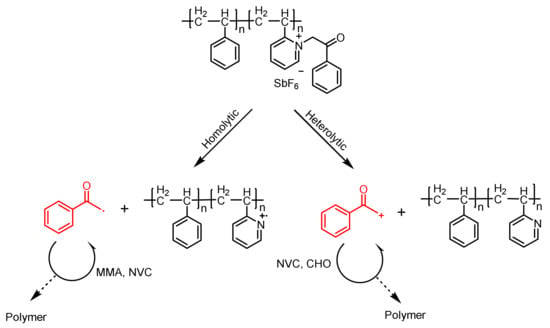
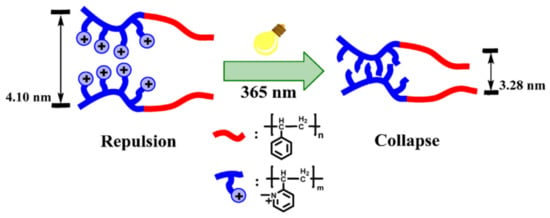
Phenacyl Ammonium Salts
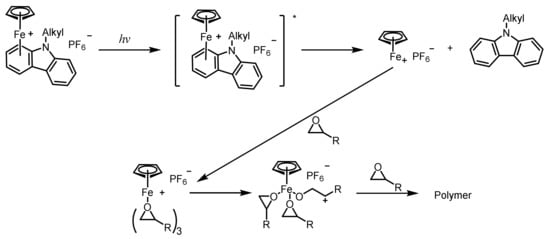
2.6. Ferrocenium Salts
3. Nonionic Photoinitiators for Cationic Polymerization
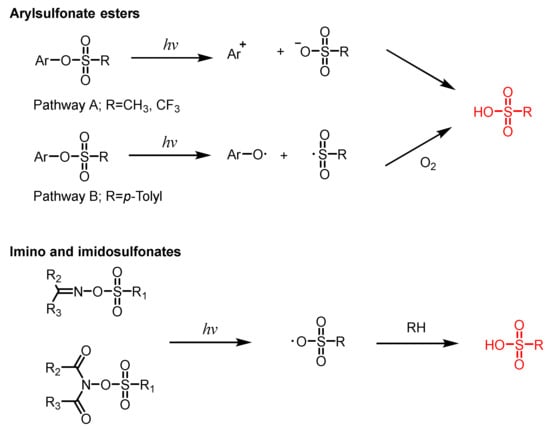
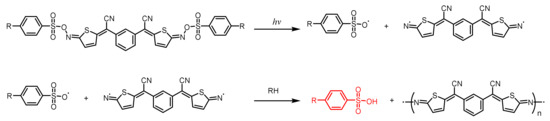
This entry is adapted from the peer-reviewed paper 10.3390/polym15112524
References
- Crivello, J.V. The discovery and development of onium salt cationic photoinitiators. J. Polym. Sci. Pol. Chem. 1999, 37, 4241–4254.
- Crivello, J.V.; Lam, J. Diaryliodonium salts. A new class of photoinitiators for cationic polymerization. Macromolecules 1977, 10, 1307–1315.
- Ortyl, J.; Popielarz, R. New photoinitiators for cationic polymerization. Polimery 2012, 57, 510–517.
- Park, C.H.; Takahara, S.; Yamaoka, T. The participation of the anion and alkyl substituent of diaryliodonium salts in photo-initiated cationic polymerization reactions. Polym. Adv. Technol. 2006, 17, 156–162.
- Pobiner, H. Spectrophotometric determinations of aryldiazonium salts of complex halides used as photoinitiators in uv-cured epoxide systems. Anal. Chim. Acta. 1978, 96, 153–163.
- Yağci, Y.; Reetz, I. Externally stimulated initiator systems for cationic polymerization. Prog. Polym. Sci. 1998, 23, 1485–1538.
- Hartwig, A.; Harder, A.; Lühring, A.; Schröder, H. (9-Oxo-9H-fluoren-2-yl)-phenyl-iodonium hexafluoroantimonate (V)—A photoinitiator for the cationic polymerisation of epoxides. Eur. Polym. J. 2001, 37, 1449–1455.
- Zivic, N.; Bouzrati-Zerrelli, M.; Villotte, S.; Morlet-Savary, F.; Dietlin, C.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevee, J. A novel naphthalimide scaffold based iodonium salt as a one-component photoacid/photoinitiator for cationic and radical polymerization under LED exposure. Polym. Chem. 2016, 7, 5873–5879.
- Topa, M.; Hola, E.; Galek, M.; Petko, F.; Pilch, M.; Popielarz, R.; Morlet-Savary, F.; Graff, B.; Lalevée, J.; Ortyl, J. One-component cationic photoinitiators based on coumarin scaffold iodonium salts as highly sensitive photoacid generators for 3D printing IPN photopolymers under visible LED sources. Polym. Chem. 2020, 11, 5261–5278.
- Petko, F.; Galek, M.; Hola, E.; Popielarz, R.; Ortyl, J. One-Component Cationic Photoinitiators from Tunable Benzylidene Scaffolds for 3D Printing Applications. Macromolecules 2021, 54, 7070–7087.
- Crivello, J.; Lam, J. Photoinitiated cationic polymerization by diarylchloronium and diarylbromonium salts. J. Polym. Sci. Polym. Lett. Ed. 1978, 16, 563–571.
- Crivello, J.; Lam, J. Tethered sulfonium salt photoinitiators for free radical polymerization. Synth. Commun. 1979, 9, 151–156.
- Crivello, J.; Lam, J. Photoinitiated cationic polymerization with triarylsulfonium salts. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 3231–3253.
- Crivello, J.V. Benzophenothiazine and benzophenoxazine photosensitizers for triarylsulfonium salt cationic photoinitiators. J. Polym. Sci. Pol. Chem. 2008, 46, 3820–3829.
- Crivello, J.V. Redox initiated cationic polymerization: Reduction of triarylsulfonium salts by silanes. Silicon 2009, 1, 111–124.
- Jin, M.; Wu, X.; Malval, J.P.; Wan, D.; Pu, H. Dual roles for promoting monomers to polymers: A conjugated sulfonium salt photoacid generator as photoinitiator and photosensitizer in cationic photopolymerization. J. Polym. Sci. Polym. Chem. 2016, 54, 2722–2730.
- Crivello, J.; Lam, J. Photoinitiated cationic polymerization by dialkyl-4-hydroxyphenylsulfonium salts. J. Polym. Sci. Polym. Chem. Ed. 1980, 18, 1021–1034.
- Crivello, J.; Lam, J. Complex triarylsulfonium salt photoinitiators. I. The identification, characterization, and syntheses of a new class of triarylsulfonium salt photoinitiators. J. Polym. Sci. Polym. Chem. Ed. 1980, 18, 2677–2695.
- Klikovits, N.; Knaack, P.; Bomze, D.; Krossing, I.; Liska, R. Novel photoacid generators for cationic photopolymerization. Polym. Chem. 2017, 8, 4414–4421.
- Zhang, B.; Li, T.; Kang, Y. Synthesis and characterization of triarylsulfonium salts as novel cationic photoinitiators for UV-photopolymerization. Res. Chem. Intermed. 2017, 43, 6617–6625.
- Yu, J.; Pan, H.; Jiang, S.L.; Gao, Y.J.; Sun, F. Synthesis and performances of polysiloxane-modified 5-arylthianthrenium salt cationic photoinitiators with self-floating capability. Eur. Polym. J. 2017, 97, 338–346.
- Chen, S.; Cao, C.; Shen, X.; Qiu, Y.; Kuang, C.; Wan, D.; Jin, M. One/two-photon sensitive sulfonium salt photoinitiators based on 1, 3, 5-triphenyl-2-pyrazoline. Eur. Polym. J. 2021, 153, 110525.
- Wu, X.; Jin, M.; Xie, J.; Malval, J.P.; Wan, D. Molecular Engineering of UV/Vis Light-Emitting Diode (LED)-Sensitive Donor–π–Acceptor-Type Sulfonium Salt Photoacid Generators: Design, Synthesis, and Study of Photochemical and Photophysical Properties. Chem. Eur. J. 2017, 23, 15783–15789.
- Wu, X.; Jin, M.; Xie, J.; Malval, J.P.; Wan, D. One/two-photon cationic polymerization in visible and near infrared ranges using two-branched sulfonium salts as efficient photoacid generators. Dyes Pigments 2016, 133, 363–371.
- Wu, X.; Malval, J.-p.; Wan, D.; Jin, M. D-π-A-type aryl dialkylsulfonium salts as one-component versatile photoinitiators under UV/visible LEDs irradiation. Dyes Pigments 2016, 132, 128–135.
- Jin, M.; Hong, H.; Xie, J.; Malval, J.-P.; Spangenberg, A.; Soppera, O.; Wan, D.; Pu, H.; Versace, D.-L.; Leclerc, T. π-conjugated sulfonium-based photoacid generators: An integrated molecular approach for efficient one and two-photon polymerization. Polym. Chem. 2014, 5, 4747–4755.
- Xia, R.; Malval, J.-P.; Jin, M.; Spangenberg, A.; Wan, D.; Pu, H.; Vergote, T.; Morlet-Savary, F.; Chaumeil, H.; Baldeck, P. Enhancement of acid photogeneration through a para-to-meta substitution strategy in a sulfonium-based alkoxystilbene designed for two-photon polymerization. Chem. Mater. 2012, 24, 237–244.
- Meng, X.Y.; Li, L.J.; Huang, Y.X.; Deng, X.; Liu, X.X.; Li, Z.Q. Upconversion nanoparticle-assisted cationic and radical/cationic hybrid photopolymerization using sulfonium salts. Polym. Chem. 2021, 12, 7005–7009.
- Crivello, J.V.; Kong, S. Synthesis and characterization of second-generation dialkylphenacylsulfonium salt photoinitiators. Macromolecules 2000, 33, 825–832.
- Kaya, K.; Kreutzer, J.; Yagci, Y. Diphenylphenacyl sulfonium salt as dual photoinitiator for free radical and cationic polymerizations. J. Polym. Sci. Pol. Chem. 2018, 56, 451–457.
- Zhu, Y.; Xu, D.; Zhang, Y.; Zhou, Y.; Yagci, Y.; Liu, R. Phenacyl Phenothiazinium Salt as a New Broad-Wavelength-Absorbing Photoinitiator for Cationic and Free Radical Polymerizations. Angew. Chem. Int. Ed. 2021, 60, 16917–16921.
- Zhu, Y.; Li, L.; Zhang, Y.C.; Ou, Y.; Zhang, J.Y.; Yagci, Y.; Liu, R. Broad wavelength sensitive coumarin sulfonium salts as photoinitiators for cationic, free radical and hybrid photopolymerizations. Prog. Org. Coat. 2023, 174, 107272.
- Abu-Abdoun, I.I. Cationic photopolymerization of cyclohexene oxide. Eur. Polym. J. 1992, 28, 73–78.
- Kasapoglu, F.; Aydin, M.; Arsu, N.; Yagci, Y. Photoinitiated polymerization of methyl methacrylate by phenacyl type salts. J. Photochem. Photobiol. A 2003, 159, 151–159.
- Takata, T.; Takuma, K.; Endo, T. Photoinitiated cationic polymerization of epoxide with phosphonium salts as novel photolatent initiators. Makromol. Chem. Rapid Commun. 1993, 14, 203–206.
- Tskuma, K.; Takata, T.; Endo, T. Latent cationic initiator: Photoinitiated polymerization of epoxides and vinyl monomers with phosphonium salts. J. Photopolym. Sci. Tecnol. 1993, 6, 67–74.
- Yaḡci, Y.; Kornowski, A.; Schnabel, W. N-alkoxy-pyridinium and N-alkoxy-quinolinium salts as initiators for cationic photopolymerizations. J. Polym. Sci. Pol. Chem. 1992, 30, 1987–1991.
- Taskin, O.S.; Erel-Goktepe, I.; Khan, M.; Alyaan, A.; Pispas, S.; Yagci, Y. Polystyrene-b-poly(2-vinyl phenacyl pyridinium) salts as photoinitiators for free radical and cationic polymerizations and their photoinduced molecular associations. J. Photochem. Photobiol. A 2014, 285, 30–36.
- Durmaz, Y.Y.; Zaim, Ö.; Yagci, Y. Diethoxy-azobis (pyridinium) Salt as Photoinitiator for Cationic Polymerization: Towards Wavelength Tunability by Cis–Trans Isomerization. Macromol. Rapid Comm. 2008, 29, 892–896.
- Karal, O.; Önen, A.; Yaǧcı, Y. Polymeric pyridinium salts as photoinitiators for cationic polymerization. Polymer 1994, 35, 4694–4696.
- Zhu, Q.Q.; Schnabel, W. Cationic polymerization initiated by onium ions. Eur. Polym. J. 1997, 33, 1325–1331.
- Kasapoglu, F.; Onen, A.; Bicak, N.; Yagci, Y. Photoinitiated cationic polymerization using a novel phenacyl anilinium salt. Polymer 2002, 43, 2575–2579.
- Kreutzer, J.; Demir, K.D.; Yagci, Y. Synthesis and characterization of a double photochromic initiator for cationic polymerization. Eur. Polym. J. 2011, 47, 792–799.
- Yonet, N.; Bicak, N.; Yagci, Y. Photoinitiated cationic polymerization of cyclohexene oxide by using phenacyl benzoylpyridinium salts. Macromolecules 2006, 39, 2736–2738.
- Yonet, N.; Bicak, N.; Yurtsever, M.; Yagci, Y. Spectroscopic and theoretical investigation of capillary-induced keto–enol tautomerism of phenacyl benzoylpyridinium-type photoinitiators. Polym. Int. 2007, 56, 525–531.
- Li, L.J.; Wan, M.D.; Li, Z.Q.; Luo, Y.C.; Wu, S.F.; Liu, X.X.; Yagci, Y. Coumarinacyl Anilinium Salt: A Versatile Visible and NIR Photoinitiator for Cationic and Step-Growth Polymerizations. ACS Macro Lett. 2023, 12, 263–268.
- Lohse, F.; Zweifel, H. Recent advances in cationic photopolymerization of epoxides. Adv. Polym. Sci. 1986, 78, 69–76.
- Tao, W.; Yuli, H.; Shujian, S. Studies on the photosensitivity of cationic polymerization photoinitiator BF4. Chem. J. Chin. Univ. 2003, 24, 735–738.
- Nesmeyanov, A.; Vol’Kenau, N.; Bolesova, I. The interaction of ferrocene and its derivatives with aromatic compounds. Tetrahedron Lett. 1963, 4, 1725–1729.
- Li, Z.Q.; Li, M.; Li, G.; Chen, Y.; Wang, X.; Wang, T. Naphthoxy bounded ferrocenium salts as cationic photoinitiators for epoxy photopolymerization. Int. J. Photoenergy 2009, 2009, 981068.
- Tao, W.; Pingyu, W.; Lijun, M. Synthesis and characterization of alkoxy and phenoxy-substituted ferrocenium salt cationic photoinitiators. Chin. J. Chem. Eng. 2006, 14, 806–809.
- Wang, T.; Chen, J.W.; Li, Z.Q.; Wan, P.Y. Several ferrocenium salts as efficient photoinitiators and thermal initiators for cationic epoxy polymerization. J. Photochem. Photobiol. A 2007, 187, 389–394.
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. The carbazole-bound ferrocenium salt as a specific cationic photoinitiator upon near-UV and visible LEDs (365–405 nm). Polym. Bull. 2016, 73, 493–507.
- Zhang, J.; Xiao, P.; Dietlin, C.; Campolo, D.; Dumur, F.; Gigmes, D.; Morlet-Savary, F.; Fouassier, J.P.; Lalevée, J. Cationic photoinitiators for near UV and visible LEDs: A particular insight into one-component systems. Macromol. Chem. Phys. 2016, 217, 1214–1227.
- Ikbal, M.; Jana, A.; Singh, N.P.; Banerjee, R.; Dhara, D. Photoacid generators (PAGs) based on N-acyl-N-phenylhydroxylamines for carboxylic and sulfonic acids. Tetrahedron 2011, 67, 3733–3742.
- Fouassier, J.P.; Burr, D. Triplet state reactivity of α-sulfonyloxy ketones used as polymerization photoinitiators. Macromolecules 1990, 23, 3615–3619.
- Ikbal, M.; Banerjee, R.; Atta, S.; Dhara, D.; Anoop, A.; Singh, N.P. Synthesis, photophysical and photochemical properties of photoacid generators based on N-hydroxyanthracene-1, 9-dicarboxyimide and their application toward modification of silicon surfaces. J. Org. Chem. 2012, 77, 10557–10567.
- Shirai, M.; Okamura, H. i-Line sensitive photoacid generators for UV curing. Prog. Org. Coat. 2009, 64, 175–181.
- Martin, C.J.; Rapenne, G.; Nakashima, T.; Kawai, T. Recent progress in development of photoacid generators. J. Photochem. Photobiol. C Photochem. Rev. 2018, 34, 41–51.
- Zivic, N.; Kuroishi, P.K.; Dumur, F.; Gigmes, D.; Dove, A.P.; Sardon, H. Recent advances and challenges in the design of organic photoacid and photobase generators for polymerizations. Angew. Chem. Int. Ed. 2019, 58, 10410–10422.
- Sun, X.; Jin, M.; Wu, X.; Pan, H.; Wan, D.; Pu, H. Bis-substituted thiophene-containing oxime sulfonates photoacid generators for cationic polymerization under UV–visible LED irradiation. J. Polym. Sci. Pol. Chem. 2018, 56, 776–782.
