Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The market offers supplements containing food plant-derived molecules (e.g., primary and secondary metabolites, vitamins, and fibers), microbes (probiotics), and microbial-derived fractions (postbiotics). They can control lipid and carbohydrate metabolism, reduce appetite (interacting with the central nervous system) and adipogenesis, influence intestinal microbiota activity, and increase energy expenditure. Unfortunately, the copious choice of products and different legislation on food supplements worldwide can confuse consumers.
- antiobesity
- food-derived moieties
- antiobesity phytochemicals
- prebiotics
- microbial-derived moieties
- probiotics
- metabiotic
- parabiotic
- postbiotic
1. Obesity
The body mass index (BMI) values body fat based on a person’s weight and height. A person whose BMI is over 25 is considered to be overweight, and obese if it is over 30 (Figure 1). Family genetics (a propensity to accumulate fat), psychological factors, and lifestyle (poor exercise or dietary habits) can result in obesity [1]. In living organisms, lipids and fatty acids are formed from glucose. Successively, fatty acids are esterified into triglycerides and stored in adipose tissue. Amylases and glucosidases are the key enzymes that metabolize carbohydrates into glucose [2]. Increased glucose levels determine the insulin release from pancreatic cells and induce glycogenesis, glycolysis, and lipogenesis [3].
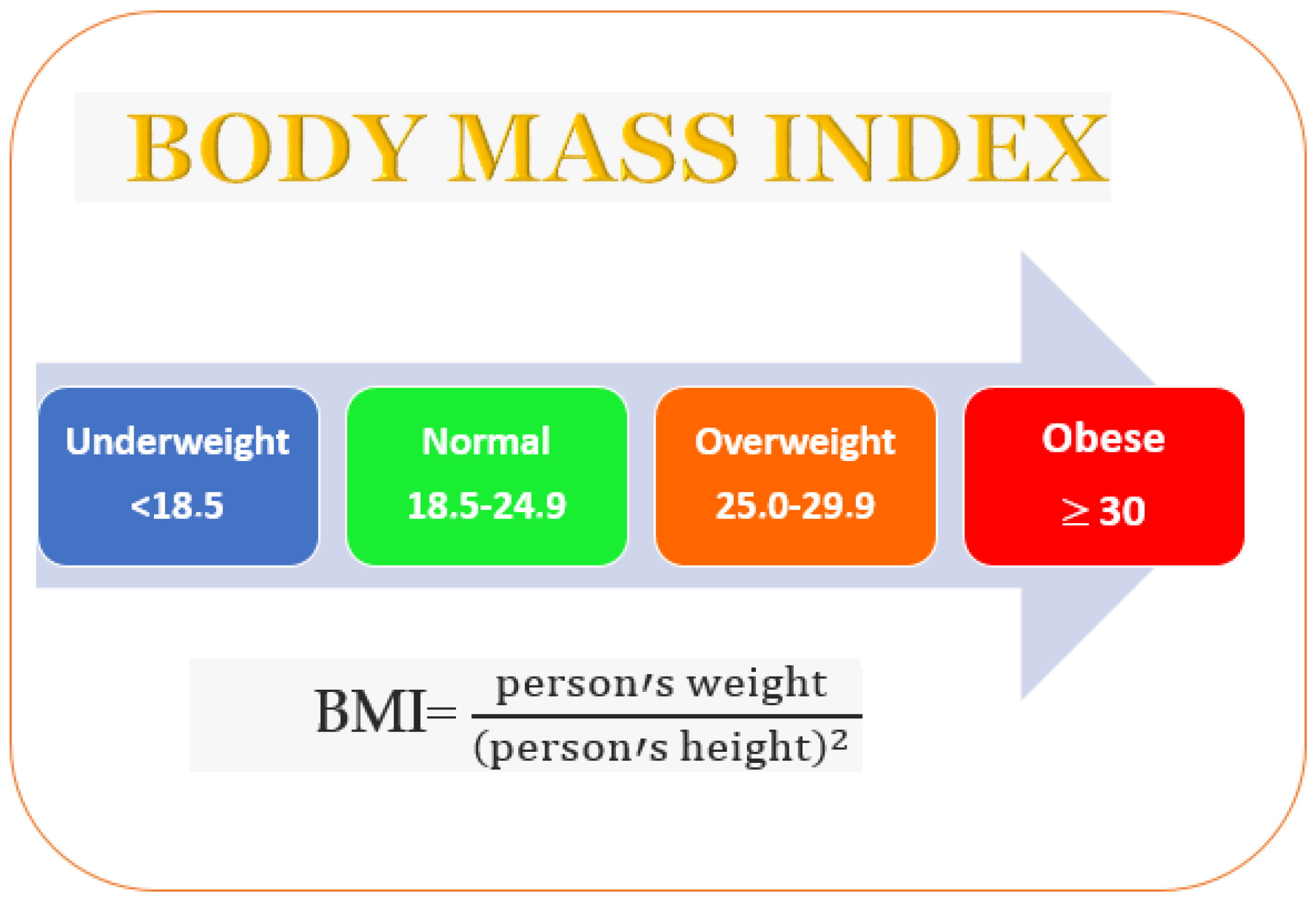
Figure 1. Overweight incidence evaluated by the body mass index.
Pancreatic lipase is a critical enzyme in dietary fat digestion. It reduces the fat deposition into adipose tissue and controls the digestion and absorption of triglycerides [4]. This lipase is upregulated by glucagon and epinephrine and downregulated by insulin [5]. Adipose tissue regulates obesity. Adipocytes act as energy storage, detect energy demands, and produce paracrine factors to regulate other metabolic tissues. In obesity, adipose tissue becomes severely dysfunctional, does not store excess energy, causes ectopic fat deposition [6], enhances the levels of free fatty acid metabolites (e.g., ceramide, long-chain fatty acyl Coenzyme A, and di-acyl glycerol) [7], and regulates insulin resistance by constraining the protein-kinase B (PKB) pathway [8].
Hyperinsulinemia increases the ATP level and downregulates the AMP-activated protein kinase (AMPK) pathway [9]. In obesity, preadipocyte differentiation into mature adipocytes is promoted [10], as is the production of inflammatory cytokines (such as the Tumor necrosis factor alpha (TNF-α) and some interleukins such as IL-6, IL-1, and IL-18) [11]. TNF-α downregulates insulin sensitivity (improving IκB kinase/NF-κB signaling), glucose uptake (preventing the GLUT-4 transporter), the 5′ AMP-activated protein kinase (AMPK) pathway, lipogenesis (reducing PPARγ expression), and increases lipolysis [12]. Some hormones (e.g., leptin, insulin, adiponectin, and ghrelin) are involved in the etiopathogenesis of obesity. Leptin is released by white adipose tissue (WAT) and regulates the brain–gut axis. It controls appetite and metabolism by impeding the synthesis and release of neuropeptide Y in the arcuate nucleus. The leptin isoform b (LEP-Rb) regulates the energy balance and body mass in the ventromedial hypothalamic nucleus, arcuate nucleus, lateral hypothalamic nuclei, and dorsomedial hypothalamic nucleus and decreases appetite [13]. Insulin (secreted from pancreatic beta cells) converts signals to the brain and decreases food intake (over the long term) and rapid energy outflow. Brain insulin signaling regulates systemic and organ-specific metabolism, often in a complementary manner [14] (Figure 2). Signals from leptin and insulin communicate to reduce food and energy intake [15], the metabolisms of carbohydrates and lipids [16], fatty acid oxidation, and glucose uptake in the skeletal muscle and liver [17]. Adiponectin can activate the adenosine monophosphate-activated protein kinase (AMPK) and decrease acetyl CoA carboxylase and malonyl CoA activities [18][19].
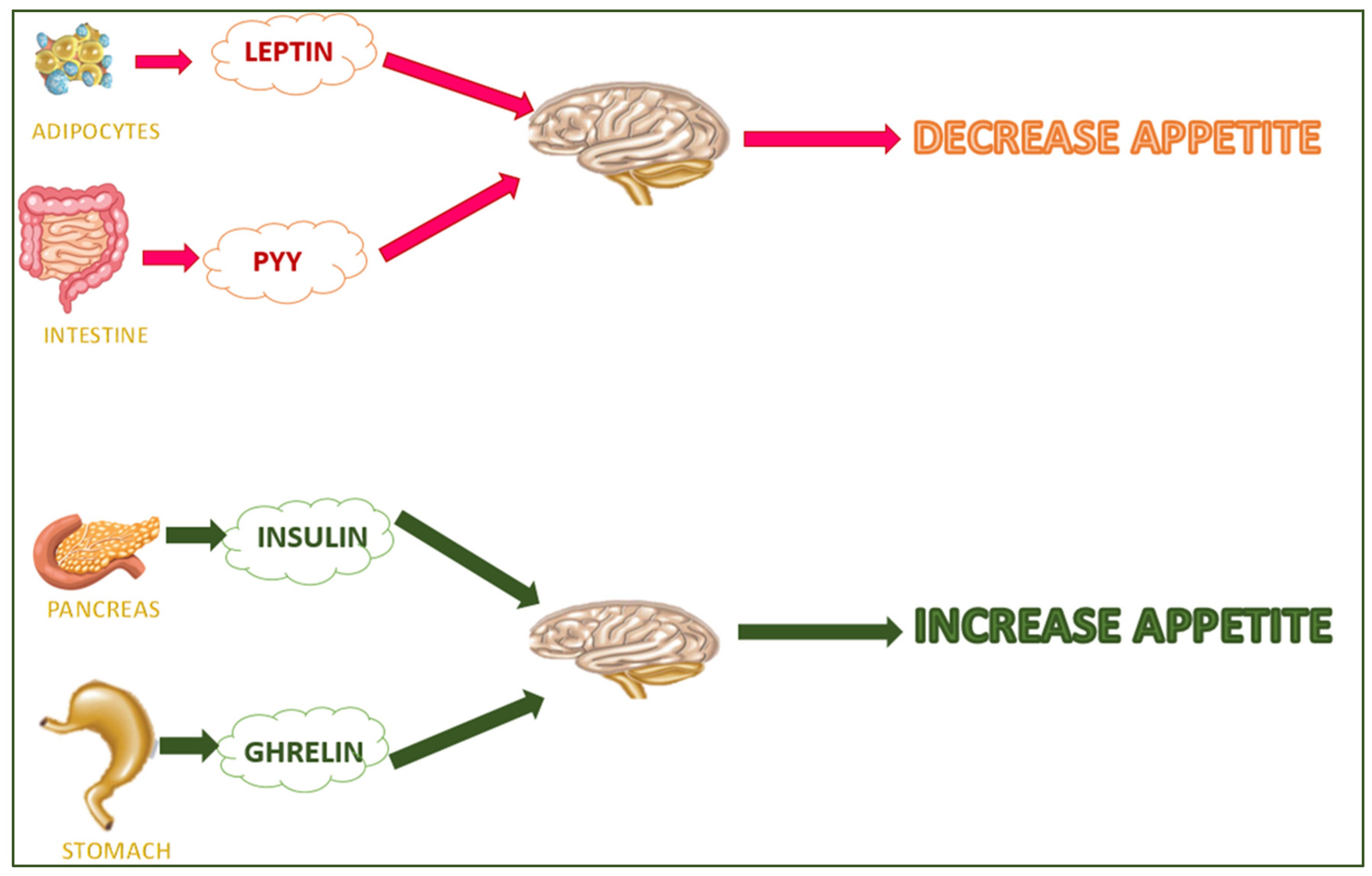
Figure 2. Hunger/satiety-regulating hormones.
Adiponectin is secreted from adipose tissue and controls energy homeostasis and the metabolisms of carbohydrates and lipids [16]. It improves the fatty acid oxidation, hepatic insulin activity, and glucose uptake in the skeletal muscle and liver [17]. Adiponectin can activate the adenosine monophosphate-activated protein kinase (AMPK) and decrease acetyl CoA carboxylase and malonyl CoA activities [18][19]. The stomach secretes ghrelin (the hunger hormone), which stimulates food intake and adiposity [20]. Finally, endoplasmic reticulum stress can affect insulin resistance, activating the Jun N-terminal kinase (JNK) and inhibitory kappa B kinase (IKK) pathways [21].
2. Supplement Regulation
Urbanization and income growth worldwide have increased the demand for products that control weight management. This segment is expected to grow significantly in the coming period due to the prevalence of obesity among adults and children worldwide linked to changing food habits [22]. The global dietary supplements market will probably reach 327.4 billion USD by 2030. Dietary supplements are regulated differently around the world. In the USA, they are regulated as food by the FDA (Food and Drug Administration) under the DSHEA of 1994 (Dietary Supplement Health and Education Act) [23]. In the United Kingdom, food supplements are regulated by the Department of Health and Social Care (England), Food Standards Scotland (Scotland), Welsh Government (Wales), and Food Standards Agency (Northern Ireland). They are defined as “food whose purpose is to supplement the normal diet and which is a concentrated source of a vitamin or mineral or other substance with a nutritional or physiological effect, alone or in combination and is sold in dose form” [24]. In other jurisdictions, they are considered to be therapeutic goods, food supplements, prescription medicines, or controlled substances [25]. In Italy, the Directive 2002/46/EC and Legislative Decree 21 May 2004 n. 169 regulate dietary supplements as “food products that can supplement the common diet. They are a source concentrate of nutrients, such as vitamins and minerals, or other substances having an effect nutritional or physiological, in particular—but not exclusively—amino acids, essential fatty acids, probiotic microorganisms, fibers, and extracts of vegetable origin, both mono-compound and multi-compound” [26]. The uneven legislation on the marketing of these products around the world can confuse consumers. It is hoped that convergence on this matter can be achieved as soon as possible.
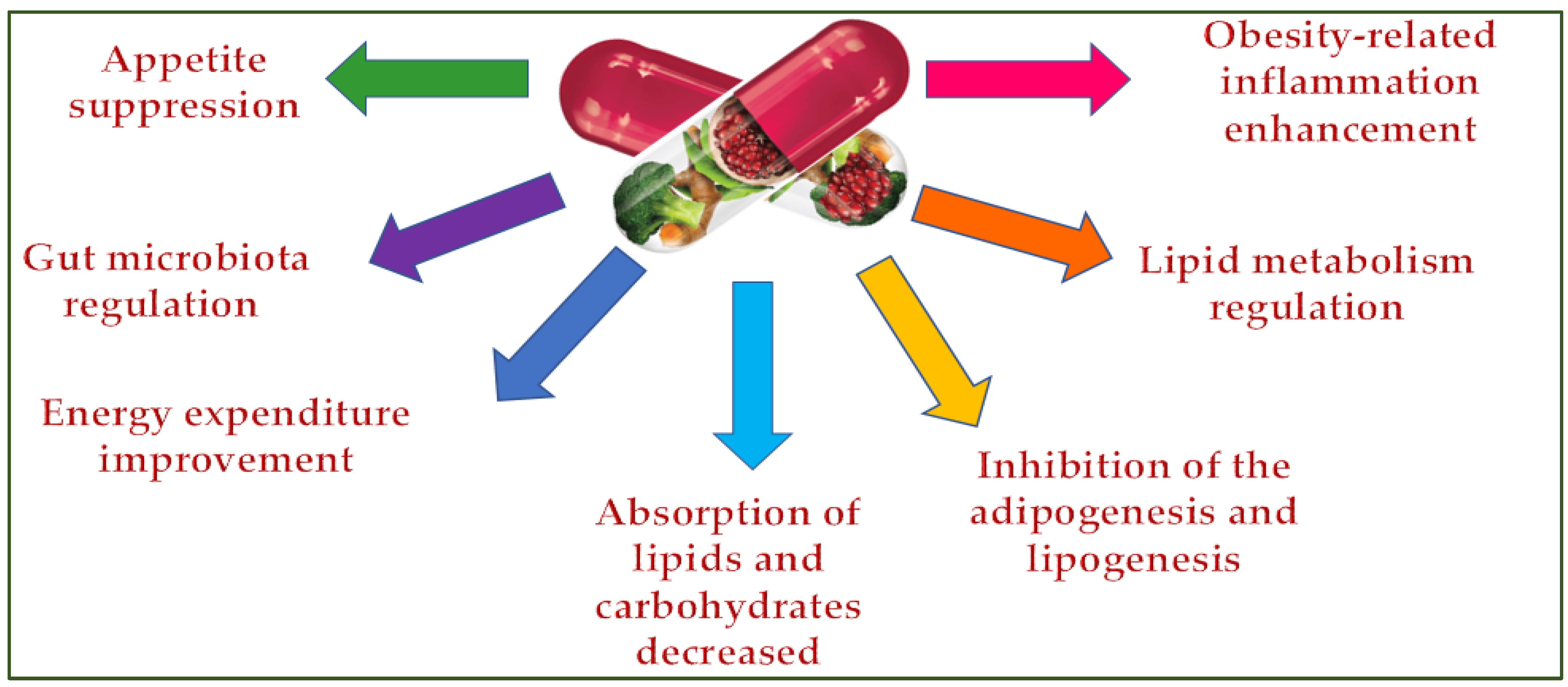
3. Weight Management Supplements
Dietary supplements can control being overweight by inhibiting the appetite [27], lipid and carbohydrate absorption [28], adipogenesis and lipogenesis [29], regulating lipid metabolism and the gut microbiota [30], and improving energy consumption [31] and obesity-related inflammation (Figure 3) [32].

Figure 3. Dietary supplements’ antiobesity action mechanisms.
3.1. Plants Extract in Supplements for Weight Control Management
Usually, weight loss supplements are multi-ingredient preparations (an average of 10 ingredients are enclosed) [33]. It is difficult to determine their effects on the body due to the recipes’ complexity and different dosages, extract types, and administration times used in studies. Some food or medicinal plants are employed in weight control treatments. Their effects are mainly linked to secondary metabolites (e.g., polyphenols and saponins, etc.) [34][35], unsaturated fatty acids, and fibers. Natural products that are in used in weight control management include green tea, garcinia cambogia, turmeric, ginger, coffee, chili pepper, spirulina, licorice, hibiscus sabdariffa, white bean, and yerba maté, etc.
Green tea (GT) extract decreases waist circumference (WMD: −2.06 cm) when GT of ≥800 mg/day for <12 weeks or GT of <500 mg/day for 12 weeks is consumed [36]. The consumption of green tea extract for up 14 weeks decreases body weight (BW: 1.8 kg) and body mass index (BMI: 0.65 kg/m2) [36]. Unfortunately, some studies have reported that green tea extract can cause liver damage [37][38]. Dexaprine (a multi-ingredient supplement with green tea extract) has caused some consumers emesis, anxiety, and tachycardia [39]. The Linea Detox (with green tea extract) has produced anaphylactic reactions [40].
3.2. Dietary Supplements Able to Decrease the Appetite
Appetite control can reduce weight gain [41]. They can contain grains (e.g., wheat, oats, corn, rice, rye, or barley) [42], prebiotics (e.g., fructosan and inulin) [43], secondary metabolites such as saponins (e.g., pregnane glycosides and stavarosides) [44], methylxanthines (e.g., caffeine, theobromine, and theophylline) [45], and hydrolyzed yeast proteins [46].
3.3. Dietary Supplements Able to Interact with the Central Nervous System
Some supplements can promote antiobesogenic effects, interacting with the central nervous system and determining the release of hormones, such as the neuropeptide Y (that can delay satiety and promote food intake), norepinephrine (that can increase lipolysis), the POMC/CART (that can regulate food consumption) [47], the melanocortins and α-melanocyte-stimulating hormone (that can regulate the appetite and are affected by leptin and insulin) [48], and serotonin (that can regulate food intake). The plant secondary metabolites that can interact with the hormones released by the central nervous system are ephedrine (that acts as a sympathomimetic agent) [49], the red ginseng’s saponins (protopanaxadiol and protopanaxatriol type that act by downregulating leptin and neuropeptide Y) [50][51], the garcinia’s hydroxy citric acids (that control the glucose and uptake of serotonin level) [52][53], the amines in citrus with aromatic rings (that improve serotonin levels) [54], and fucoxanthin isolated from brown seaweed (that impacts insulin levels) [55].
3.4. Dietary Supplements That Interact with the Hormones in the Digestive System and Adipose Tissue
Some dietary supplements suppress the appetite by regulating the secretion of hormones in the digestive system (e.g., the ghrelin in the stomach) and adipose tissue (e.g., leptin, secreted by adipocytes [56], the AMP-activated protein kinase that controls energy metabolism [57], and the carnitine palmitoyl transferase 1A and cofactor for the beta-oxidation of fatty acids that enhance the fatty acid oxidation) [58].
3.5. Prebiotics in Weight Control Supplements
Prebiotics are non-viable food components (e.g., non-digestible carbohydrates, peptides, proteins, and lipids) [59] that can positively impact beneficial bacteria’s activity (e.g., Lactobacillus and Bifidobacterium) and/or growth in the gut microbiota [60]. They are not hydrolyzed by gastric acidity and mammalian enzymes. Moreover, prebiotics do not get absorbed into the gastrointestinal tract, are fermented by the gut microbiota, and are beneficial to a host’s health [61]. The prebiotic, non-digestible carbohydrates include resistant starch, non-starch polysaccharides, and oligosaccharides composed of three–nine sugar units [62][63], which endogenous enzymes cannot hydrolyze [64]. By imitating intestinal binding sites, some prebiotics impede the pathogenic microbiota’s adhesion to the gastrointestinal tract [65]. These prebiotics can modulate the immune system by upregulating interleukins and immunoglobulins, downregulating proinflammatory interleukins [66][67], and improving short-chain fatty acids’ (SCFAs) production [68]. The SCFAs improve the intestinal barrier integrity, are an essential indicator of bacterial fermentation in the colon [69], protect against inflammation, regulate mucus production [70], and constrain obesity [71].
3.6. Probiotics in Weight Control Supplements
Probiotics are live microorganisms that affect human health when consumed adequately [72]. They control being overweight, enhancing the gut barrier function, decreasing metabolic endotoxemia, systematic inflammation, gut permeability, energy hemostasis, and appetite regulation. They can deconjugate the bile acids interfering with lipid absorption, increase SCFAs, and stimulate intestinal peptide synthesis [73][74][75]. The probiotic L. rhamnosus GG strain can constrain obesity via the upregulation of adiponectins [76]. A mix containing Bifidobacterium, Lactococcus, and Propionibacterium showed a significant reduction in the total body and visceral adipose tissue [77].
3.7. Symbiotics in Weight Control Supplements
Synbiotics are “a mixture comprising live microorganisms and substrate(s) utilized by host microorganisms that confer a health benefit on the host” [78]. The complex mixtures of bacterial strains and different dosages of prebiotic fibers in symbiotics can modulate the metabolic activity in the intestine, upregulate microbiota development, short-chain fatty acid, carbon disulfides, ketones, and methyl acetate concentrations, decrease pathogens, and inactivate nitrosamines and other cancerogenic substances [79].
3.8. Postbiotics in Weight Control Supplements
Postbiotics are products (microbial cells or cellular factors that have been attenuated with or without metabolites) or metabolites produced by bacteria or liberated after bacterial lysis, which have a beneficial role in human health [80][81]. Gut bacteria secrete low-molecular-weight metabolites that regulate their growth, promote cell-to-cell communication, and protect against environmental stresses [82][83][84]. The Lactobacillus, Bacillus, Bifidobacterium, Faecalibacterium, and Streptococcus genera can produce postbiotics [85][86]. These postbiotics emulate probiotics’ actions and have a better shelf-life, easier packaging, and minor transport requirements. SCFA, enzymes, peptides, vitamins, and teichoic acids exemplify postbiotics [87]. Acetate, propionate, and butyrate are the most rapresentative SCFAs [87][88]. Butyrate and propionate can positively downregulate the gut hormones and decrease food intake [89]. Acetate acts as a lipogenic substrate propionate that can moderate lipogenesis by downregulating the fatty acid synthase (in the liver). Therefore, the acetate/propionate ratio is crucial for de novo lipogenesis [90].
This entry is adapted from the peer-reviewed paper 10.3390/molecules28145357
References
- Fitzgerald, M.P.; Hennigan, K.; Gorman, C.S.O.; Mccarron, L. Obesity, diet and lifestyle in 9-year-old children with parentally reported chronic diseases: Findings from the growing up in Ireland longitudinal child cohort study. Ir. J. Med. Sci. 2018, 188, 29–34.
- Spínola, V.; Castilho, P.C. Assessing the In Vitro Inhibitory Effects on Key Enzymes Linked to Type-2 Diabetes and Obesity and Protein Glycation by Phenolic Compounds of Lauraceae Plant Species Endemic to the Laurisilva Forest. Molecules 2021, 26, 2023.
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Fairus, A.; Ima-Nirwana, S. Animal models of metabolic syndrome: A review. Nutr. Metab. 2016, 13, 65.
- Pirahanchi, Y.; Anoruo, M.D.; Sharma, S. Biochemistry, Lipoprotein Lipase; StatPearls Publishing: Treasure Island, FL, USA, 2020.
- Zhang, D.; Wei, Y.; Huang, Q.; Chen, Y.; Zeng, K.; Yang, W.; Chen, J.; Chen, J. Important Hormones Regulating Lipid Metabolism. Molecules 2022, 27, 7052.
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358.
- Heilbronn, L.K.; Rood, J.; Janderova, L.; Albu, J.B.; Kelley, D.E.; Ravussin, E.; Smith, S.R. Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J. Clin. Endocrinol. Metab. 2004, 89, 1844–1848.
- Greenfield, J.R.; Campbel, L.V. Insulin resistance and obesity. Clin. Dermatol. 2004, 22, 289–295.
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814.
- Pellegrinelli, V.; Carobbio, S.; Vidal-Puig, A. Adipose tissue plasticity: How fat depots respond differently to pathophysiological cues. Diabetologia 2016, 59, 1075–1088.
- Ye, J. Mechanisms of insulin resistance in obesity. Front. Med. 2013, 7, 14–24.
- Singh, P.; Rai, S.N. Factors affecting obesity and its treatment. Obes. Med. 2019, 16, 100140.
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887.
- Scherer, T.; Sakamoto, K.; Buettner, C. Brain Insulin Signalling in Metabolic Homeostasis and Disease. Nat. Rev. Endocrinol. 2021, 17, 468–483.
- Bhardwaj, M.; Yadav, P.; Vashishth, D.; Sharma, K.; Kumar, A.; Chahal, J.; Dalal, S.; Kataria, S.K. A Review on Obesity Management through Natural Compounds and a Green Nanomedicine-Based Approach. Molecules 2021, 26, 3278.
- Diep Nguyen, T.M. Adiponectin: Role in physiology and pathophysiology. Int. J. Prev. Med. 2020, 11, 136.
- Xu, W.; Tian, M.; Zhou, Y. The relationship between insulin resistance, adiponectin and C-reactive protein and vascular endothelial injury in diabetic patients with coronary heart disease. Exp. Ther. Med. 2018, 16, 2022–2026.
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295.
- Gar, C.; Thorand, B.; Herder, C.; Sujana, C.; Heier, M.; Meisinger, C.; Peters, A.; Koenig, W.; Rathmann, W.; Roden, M.; et al. Association of circulating MR-proADM with all-cause and cardiovascular mortality in the general population: Results from the KORA F4 cohort study. PLoS ONE 2022, 17, e0262330.
- Abizaid, A. Stress and obesity: The ghrelin connection. J. Neuroendocrinol. 2019, 31, e12693.
- Zhou, Y.; Murugan, D.D.; Khan, H.; Huang, Y.; Cheang, W.S. Roles and Therapeutic Implications of Endoplasmic Reticulum Stress and Oxidative Stress in Cardiovascular Diseases. Antioxidants 2021, 10, 1167.
- Dietary Supplement Market. Available online: https://www.researchandmarkets.com/reports/4479727/dietary-supplements-market-size-share-and-trends (accessed on 21 March 2022).
- Dietary Supplements. Available online: https://www.fda.gov/food/dietary-supplements (accessed on 3 June 2023).
- Food Supplements. Available online: https://www.food.gov.uk/business-guidance/food-supplements (accessed on 4 July 2023).
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41.
- Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the Approximation of the Laws of the Member States Relating to Food Supplements. OJ L 183, 12.7.2002, pp. 51–57. Consolidated Version 26/07/2017. Available online: http://data.europa.eu/eli/dir/2002/46/oj (accessed on 11 November 2019).
- Shehzad, A.; Rabail, R.; Munir, S.; Jan, H.; Fernández-Lázaro, D.; Aadil, R.M. Impact of Oats on Appetite Hormones and Body Weight Management: A Review. Curr. Nutr. Rep. 2023, 12, 66–82.
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401.
- Hwang, K.A.; Hwang, Y.J.; Im, P.R.; Hwang, H.J.; Song, J.; Kim, Y.J. Platycodon grandiflorum Extract Reduces High-Fat Diet-Induced Obesity Through Regulation of Adipogenesis and Lipogenesis Pathways in Mice. J. Med. Food 2019, 22, 993–999.
- Yilmaz, B.; Bangar, S.P.; Echegaray, N.; Suri, S.; Tomasevic, I.; Manuel Lorenzo, J.; Melekoglu, E.; Rocha, J.M.; Ozogul, F. The Impacts of Lactiplantibacillus plantarum on the Functional Properties of Fermented Foods: A Review of Current Knowledge. Microorganisms 2022, 10, 826.
- Wang, J.; Li, D.; Wang, P.; Hu, X.; Chen, F. Ginger prevents obesity through regulation of energy metabolism and activation of browning in high-fat diet-induced obese mice. J. Nutr. Biochem. 2019, 70, 105–115.
- Xu, L.; Li, D.; Zhu, Y.; Cai, S.; Liang, X.; Tang, Y.; Jin, S.; Ding, C. Swertiamarin supplementation prevents obesity-related chronic inflammation and insulin resistance in mice fed a high-fat diet. Adipocyte 2021, 10, 160–173.
- Sharpe, P.A.; Granner, M.L.; Conway, J.M.; Ainsworth, B.E.; Dobre, M. Availability of Weight-Loss Supplements: Results of an Audit of Retail Outlets in a Southeastern City. J. Am. Diet. Assoc. 2006, 106, 2045–2051.
- Younus, H.; Anwar, S. Prevention of Non-Enzymatic Glycosylation (Glycation): Implication in the Treatment of Diabetic Complication. Int. J. Health Sci. 2016, 10, 261–277.
- Rahmani, A.H.; Anwar, S.; Raut, R.; Almatroudi, A.; Babiker, A.Y.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A. Therapeutic Potential of Myrrh, a Natural Resin, in Health Management through Modulation of Oxidative Stress, Inflammation, and Advanced Glycation End Products Formation Using In Vitro and In Silico Analysis. Appl. Sci. 2022, 12, 9175.
- Lin, Y.; Shi, D.; Su, B.; Wei, J.; Găman, M.-A.; Macit, M.S.; Nascimento, I.J.B.D.; Guimaraes, N.S. The effect of green tea supplementation on obesity: A systematic review and dose–response metaanalysis of randomized controlled trials. Phytother. Res. 2020, 34, 2459–2470.
- Liver Tox: Clinical and Research Information on Drug-Induced Injury. Green Tea. National Institute of Diabetes and Digestive and Kidney Diseases. 2018. Available online: https://www.ncbi.nlm.nih.gov/books/ (accessed on 7 December 2020).
- Wharton, S.; Bonder, R.; Jeffery, A.; Christensen, R.A.G. The safety and effectiveness of commonly-marketed natural supplements for weight loss in populations with obesity: A critical review of the literature from 2006 to 2016. Crit. Rev. Food Sci. Nutr. 2019, 60, 1614–1630.
- Končić, M.Z. Getting More Than You Paid For: Unauthorized “Natural” Substances in Herbal Food Supplements on EU Market. Planta Med. 2018, 84, 394–406.
- Flis, P.; Mehrholz, D.; Nowicki, R.; Barańska-Rybak, W. Slim figure for high price. Urticaria due to weight loss products and performance enhancers—A review of three cases. Med. Ogólna Nauk. Zdrowiu 2015, 21, 369–371.
- Aaseth, J.; Ellefsen, S.; Alehagen, U.; Sundfør, T.M.; Alexander, J. Diets and Drugs for Weight Loss and Health in Obesity—An Update. Biomed. Pharmacother. 2021, 140, 111789.
- Appetite Suppressant. 2004. Available online: https://patents.google.com/patent/US20050214362A1/en (accessed on 18 June 2023).
- Thompson, M.S.; Yan, T.H.; Saari, N.; Sarbini, S.R. A review: Resistant starch, a promising prebiotic for obesity and weight management. Food Biosci. 2022, 50, 101965.
- Si, Y.; Sha, X.-S.; Shi, L.-L.; Wei, H.-Y.; Jin, Y.-X.; Ma, G.-X.; Zhang, J. Review on Pregnane Glycosides and Their Biological Activities. Phytochem. Lett. 2022, 47, 1–17.
- Compositions and Methods for Weight Reduction. 1998. Available online: https://patents.google.com/patent/US5945107 (accessed on 18 June 2023).
- Satiating Dietetic Product. 2001. Available online: https://patents.google.com/patent/WO2002094038A1/en (accessed on 18 June 2023).
- Al-Sayyar, A.; Hammad, M.M.; Williams, M.R.; Al-Onaizi, M.; Abubaker, J.; Alzaid, F. Neurotransmitters in Type 2 Diabetes and the Control of Systemic and Central Energy Balance. Metabolites 2023, 13, 384.
- Goit, R.K.; Taylor, A.W.; Lo, A.C.Y. The central melanocortin system as a treatment target for obesity and diabetes: A brief overview. Eur. J. Pharmacol. 2022, 924, 174956.
- Yoo, H.-J.; Yoon, H.-Y.; Yee, J.; Gwak, H.-S. Effects of Ephedrine-Containing Products on Weight Loss and Lipid Profiles: A Systematic Review and Metaanalysis of Randomized Controlled Trials. Pharmaceuticals 2021, 14, 1198.
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G.A. Effects of Saponins on Lipid Metabolism: A Review of Potential Health Benefits in the Treatment of Obesity. Molecules 2016, 21, 1404.
- Kim, J.H.; Hahm, D.H.; Yang, D.C.; Kim, J.H.; Lee, H.J.; Shim, I. Effect of crude saponin of Korean red ginseng on high-fat diet-induced obesity in the rat. J. Pharmacol. Sci. 2005, 97, 124–131.
- Anilkumar, A.T.; Manoharan, S.; Balasubramanian, S.; Perumal, E. Garcinia gummi-gutta: Phytochemicals and pharmacological pplications. BioFactors 2023, 49, 584–599.
- Chuah, L.O.; Ho, W.Y.; Beh, B.K.; Yeap, S.K. Updates on antiobesity effect of garcinia origin (−)-HCA. Evid. Based Complement. Altern. Med. 2013, 2013, 751658.
- The Regulation Of Appetite, Body Weight and Athletic Function with Materials Derived from Citrus Varieties. 1996. Available online: https://patents.google.com/patent/CA2248854C/en (accessed on 18 June 2023).
- Murakami, S.; Hirazawa, C.; Ohya, T.; Yoshikawa, R.; Mizutani, T.; Ma, N.; Moriyama, M.; Ito, T.; Matsuzaki, C. The Edible Brown Seaweed Sargassum horneri (Turner) C. Agardh Ameliorates High-Fat Diet-Induced Obesity, Diabetes, and Hepatic Steatosis in Mice. Nutrients 2021, 13, 551.
- Montalbano, G.; Mania, M.; Guerrera, M.C.; Laurà, R.; Abbate, F.; Levanti, M.; Maugeri, A.; Germanà, A.; Navarra, M. Effects of a Flavonoid-Rich Extract from Citrus sinensis Juice on a Diet-Induced Obese Zebrafish. Int. J. Mol. Sci. 2019, 20, 5116.
- Kola, B. Role of AMP-Activated Protein Kinase in the Control of Appetite. J. Neuroendocr. 2008, 20, 942–951.
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural products with antiobesity effects and different mechanisms of action. J. Agric. Food Chem. 2016, 64, 9571–9585.
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of functional food components on gut health. Crit. Rev. Food Sci. Nutr. 2019, 59, 1927–1936.
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412.
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184.
- Zdunczyk, Z. Physiological effect of low digestible oligosaccharides in diets for animals and humans. Pol. J. Food Nutr. Sci. 2004, 13, 115–130.
- Dai, F.J.; Chau, C.F. Classification and regulatory perspectives of dietary fiber. J. Food Drug Anal. 2017, 25, 37–42.
- Howlett, J.F.; Betteridge, V.A.; Champ, M.; Craig, S.A.S.; Meheust, A.; Jones, J.M. The definition of dietary fiber—Discussions at the Ninth Vahouny Fiber Symposium: Building scientific agreement. Food Nutr. Res. 2010, 54, 5750.
- Simpson, H.L.; Campbell, B.J. Review article: Dietary fibre-microbiota interactions. Aliment. Pharmacol. Ther. 2015, 42, 158–179.
- Pluta, R.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J. Gut microbiota and pro/prebiotics in Alzheimer’s disease. Aging 2020, 12, 5539–5550.
- Shokryazdan, P.; Faseleh Jahromi, M.; Navidshad, B.; Liang, J.B. Effects of prebiotics on immune system and cytokine expression. Med. Microbiol. Immunol. 2017, 206, 1–9.
- Megur, A.; Daliri, E.B.-M.; Baltriukienė, D.; Burokas, A. Prebiotics as a Tool for the Prevention and Treatment of Obesity and Diabetes: Classification and Ability to Modulate the Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 6097.
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625.
- Gaudier, E.; Rival, M.; Buisine, M.-P.; Robineau, I.; Hoebler, C. Butyrate enemas upregulate Muc genes expression but decrease adherent mucus thickness in mice colon. Physiol. Res. 2009, 58, 111–119.
- O’Keefe, S.J.D. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706.
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034.
- John, G.K.; Wang, L.; Nanavati, J.; Twose, C.; Singh, R.; Mullin, G. Dietary alteration of the gut microbiome and its impact on weight and fat mass: A systematic review and metaanalysis. Genes 2018, 9, 167.
- Guazzelli Marques, C.; de Piano Ganen, A.; Zaccaro de Barros, A.; Thomatieli Dos Santos, R.V.; Dos Santos Quaresma, M.V.L. Weight Loss Probiotic Supplementation Effect in Overweight and Obesity Subjects: A Review. Clin. Nutr. 2020, 39, 694–704.
- Musazadeh, V.; Zarezadeh, M.; Faghfouri, A.H.; Keramati, M.; Jamilian, P.; Jamilian, P.; Mohagheghi, A.; Farnam, A. Probiotics as an effective therapeutic approach in alleviating depression symptoms: An umbrella metaanalysis. Crit. Rev. Food Sci. Nutr. 2022; Online ahead of print.
- Barathikannan, K.; Chelliah, R.; Rubab, M.; Daliri, E.B.-M.; Elahi, F.; Kim, D.-H.; Agastian, P.; Oh, S.-Y.; Oh, D.H. Gut Microbiome Modulation Based on Probiotic Application for Antiobesity: A Review on Efficacy and Validation. Microorganisms 2019, 7, 456.
- Savcheniuk, O.; Kobyliak, N.; Kondro, M.; Virchenko, O.; Falalyeyeva, T.; Beregova, T. Short-term periodic consumption of multiprobiotic from childhood improves insulin sensitivity, prevents development of non-alcoholic fatty liver disease and adiposity in adult rats with glutamate-induced obesity. BMC Complement. Altern. Med. 2014, 14, 247.
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701.
- Hijová, E. Synbiotic Supplements in the Prevention of Obesity and Obesity-Related Diseases. Metabolites 2022, 12, 313.
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667.
- Aguilar-Toalá, J.; Garcia-Varela, R.; Garcia, H.; Mata-Haro, V.; González-Córdova, A.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114.
- Tomar, S.K.; Anand, S.; Sharma, P.; Sangwan, V.; Mandal, S. Role of probiotics, prebiotics, synbiotics and postbiotics in inhibition of pathogens. In The Battle against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2015; pp. 717–732.
- Zhang, J.; Du, G.-C.; Zhang, Y.; Liao, X.-Y.; Wang, M.; Li, Y.; Chen, J. Glutathione protects Lactobacillus sanfranciscensis against freeze-thawing, freeze-drying, and cold treatment. Appl. Environ. Microbiol. 2010, 76, 2989–2996.
- Netzker, T.; Fischer, J.; Weber, J.; Mattern, D.J.; König, C.C.; Valiante, V.; Schroeckh, V.; Brakhage, A.A. Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front. Microbiol. 2015, 6, 299.
- Bourebaba, Y.; Marycz, K.; Mularczyk, M.; Bourebaba, L. Postbiotics as potential new therapeutic agents for metabolic disorders management. Biomed. Pharmacother. 2022, 153, 113138.
- Chan, M.Z.A.; Liu, S.-Q. Fortifying foods with synbiotic and postbiotic preparations of the probiotic yeast, Saccharomyces boulardii. Curr. Opin. Food Sci. 2022, 43, 216–224.
- Moreno-Navarrete, J.M.; Serino, M.; Blasco-Baque, V.; Azalbert, V.; Barton, R.H.; Cardellini, M.; Latorre, J.; Ortega, F.; Sabater-Masdeu, M.; Burcelin, R.; et al. Gut Microbiota Interacts with Markers of Adipose Tissue Browning, Insulin Action and Plasma Acetate in Morbid Obesity. Mol. Nutr. Food Res. 2017, 62, 1700721.
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340.
- Jung, R.; Shetty, P.; James, W.; Barrand, M.; Callingham, B. Reduced thermogenesis in obesity. Nature 1979, 279, 322–323.
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249.
This entry is offline, you can click here to edit this entry!
