An analysis of the literature data on the electrical, thermal, mechanical, and electrochemical properties of the conventional perovskite-type cathode materials shows that lanthanum strontium manganite (La,Sr)MnO3 (LSM) fulfils all the requirements for its use in high-temperature SOFCs. However, as the temperature decreases, the use of LSM materials, which are predominantly electronic conductors with a low level of ionic conductivity, becomes unsatisfactory due to their low electrochemical activity for the oxygen reduction reaction (ORR). On the other hand, cobalt-based perovskite materials, including lanthanum strontium cobaltite ferrite (La,Sr)(Co,Fe)O3−δ (LSCF), are characterized by superior catalytic activity due to high values of both electronic and ionic conductivity.
1. Introduction
The development of renewable energy resources, including hybrid power systems [
1,
2,
3,
4], is one of the ways for sustainable progress towards decarbonization [
5,
6,
7]. Hybrid energy systems combining solid oxide fuel cells (SOFCs) with heat generators, energy storage devices, internal combustion engines, and solar cells have been intensively designed and manufactured today [
4,
8,
9,
10,
11]. The progressive trend in the development of SOFCs is to lower the operating temperature, which brings undoubted advantages on the way to the commercialization of these power sources, such as the use of cheaper materials, faster start-up, and increased lifetime due to the reduction of degradation processes. However, challenges arise at low operating temperatures related to the slowing down of electrode reaction kinetics and the increasing ohmic resistance of the electrolyte membrane, resulting in a reduction in the SOFC performance [
12,
13]. To maintain the electrochemical performance of SOFCs operating at low (LT) and intermediate (IT) temperatures at a satisfactory level, the material optimization has been considered for all construction parts of the SOFC, such as cathodes [
14,
15,
16,
17,
18,
19], anodes [
14,
19,
20,
21] and electrolytes [
19,
22,
23,
24]. The cathode has been shown to be the major contributor to the electrochemical degradation of the cell [
25]. The characterization and performance of the wide range of cathodes can be found in recent reviews [
13,
26,
27,
28,
29].
An analysis of the literature data on the electrical, thermal, mechanical, and electrochemical properties of the conventional perovskite-type cathode materials shows that lanthanum strontium manganite (La,Sr)MnO
3 (LSM) fulfils all the requirements for its use in high-temperature SOFCs [
26,
27]. However, as the temperature decreases, the use of LSM materials, which are predominantly electronic conductors with a low level of ionic conductivity, becomes unsatisfactory due to their low electrochemical activity for the oxygen reduction reaction (ORR) [
30,
31]. On the other hand, cobalt-based perovskite materials, including lanthanum strontium cobaltite ferrite (La,Sr)(Co,Fe)O
3−δ (LSCF), are characterized by superior catalytic activity [
32] due to high values of both electronic and ionic conductivity [
33]. However, these materials exhibit increased thermal and chemical expansion, which is detrimental to the long-term operation of SOFCs [
33,
34]. The poor long-term durability of high-temperature electrochemical cells is often caused by the performance degradation phenomenon of the air electrode [
35,
36,
37]. It has been found that the electrochemical performance degradation of the LSM- and LSCF-based air electrodes may include microstructural coarsening [
33,
38,
39], the electrolyte/cathode interface reactions [
33,
40,
41,
42], sulfur [
43,
44,
45] and chromium poisoning [
44,
46,
47,
48,
49], carbon deposition [
50,
51], and Sr surface segregation [
33,
35,
36,
52].
The perceived drawbacks of lanthanum strontium-based cathodes have driven research trends towards alternative solutions to improve their electrochemical performance and durability. A search of the Scopus database using the combination of the keywords “LSM”, “SOFC*” and “LSCF”, “SOFC*” (with further application of the limits of “cathode*”, “cathode materials”, “composite cathode*”, “cathode polarization”, “cathode performance”, “electrode*”, “oxygen electrode”, “electrochemical electrodes”) yielded 1141 and 1208 documents respectively for the period of 1996 for LSM and 1998 for LSCF to June of 2023.
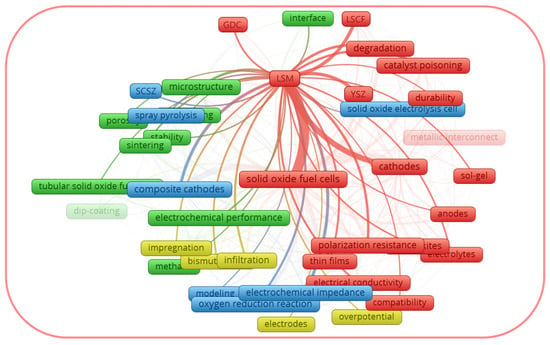
Figure 1 and
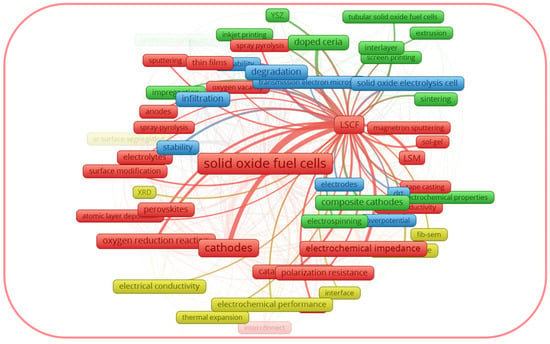
Figure 2, generated with the software package
VOSviewer version 1.6.19 [
53], considering a minimum number of occurrences equal to five author keywords, visualize the maps with topic clusters related to LSM and LSCF as cathode materials for SOFCs, respectively.
Figure 1. Thematic map of co-occurring author keywords in the Scopus dataset for (La,Sr)MnO3 (LSM).
Figure 2. Thematic map of co-occurring author keywords in the Scopus dataset for (La,Sr)(Co,Fe)O3−δ (LSCF).
From the graphical data shown in Figure 1, the author keywords of the documents considered as LSM can be divided into four clusters: the red cluster, focused on the degradation of the cells; the blue cluster, focused on the composite electrodes; the green cluster, focused on the cathode microstructure; and the yellow cluster, focused on the electrode modification using infiltration. Thus, the red cluster generalizes the drawbacks of the LSM cathode, while the other three clusters reflect the strategies to overcome them. From the graphical data shown in Figure 2, the author keywords of the documents considered as LSCF can be divided into four clusters, some of which are different from those for LSM. In the case of LSCF, the red, blue, and the green clusters generalize the LSCF performance under the operating conditions of fuel cells, electrolysis cells, and microtubular SOFCs, respectively. The yellow cluster generalizes the interfacial characterization. It is interesting to note that each cluster for LSCF includes the topic of drawbacks and their possible solutions. Thus, the above-mentioned trends justify that the improvement of the activity of LSM and LSCF electrodes remains prominent, and it is important to summarize the thematic studies, provided that the performance of LSM and LSCF has been improved.
2. Key Functional Properties of LSM and LSCF Electrode Materials: Advantages and Drawbacks
The lanthanum strontium manganite La
1−xSr
xMnO
3−δ (LSM), as a representative of complex oxides with a perovskite ABO
3 structure with rhombohedral distortions in the compositional range of 0.2 ≤ x < 0.4 [
56,
57,
58], is known to be used as a material for the fabrication of air electrodes for electrochemical devices operating at low- [
59] and intermediate temperatures [
30]. On the one hand, this is due to the high level of electronic conductivity σ
e for LSM (corresponding to 200 S cm
−1, 250 S cm
−1, and 320 S cm
−1 for La
0.8Sr
0.2MnO
3−δ (LSM20), La
0.7Sr
0.3MnO
3−δ (LSM30), and La
0.6Sr
0.4MnO
3−δ (LSM40), respectively, at 800 °C and at Po
2 = 1 bar [
60]). Secondly, due to the closest values of the coefficient of linear thermal expansion (CTE) of the LSM (e.g., for LSM20 — 11.4 × 10
−6 K
−1 in the range of 50–1000 °C, [
61]; for LSM30 — 12.2 × 10
−6 K
−1 and 13.2 × 10
−6 K
−1 in the ranges of 200–650 °C and 650–900 °C, respectively, [
62]; LSM40 — 12.7 × 10
−6 K
−1 in the range of 50–1000 °C, [
61];) to those for the solid electrolytes perspective for operating in IT-SOFCs and low-temperature SOFCs (LT-SOFCs) [
15,
22,
24,
63,
64,
65]. The CTE values can be mentioned for doped ceria (e.g., for Ce
0.8Sm
0.2O
2−δ (SDC) — 12.0 × 10
−6 K
−1 in the range of 25–1000 °C, [
66]; for Ce
0.8Gd
0.2O
2−δ (GDC) — 12.2 × 10
−6 K
−1 in the range of 50–900 °C, [
67]), (Sr,Mg)-doped LaGaO
3 (in general LSGM, e.g., for La
0.8Sr
0.2Ga
0.8Mg
0.2O
3−δ — 12.1 × 10
−6 K
−1 in the range of 25–1000 °C, [
68]), Sc
2O
3-stabilized ZrO
2 (ScSZ) (for 8ScSZ — 10.4 × 10
−6 K
−1 in the range of 30–1000 °C, [
69]). In addition, LSM electrodes offer such an important advantage as the improved stability during operation under SOFC and solid oxide electrolysis cell (SOEC) conditions compared to Fe- [
48] and Co- [
70,
71] containing electrodes.
It should be noted that the oxygen diffusion and interfacial heteroexchange parameters for LSM, which is predominantly an electron conductor, are lower than those of materials possessing high mixed oxygen ion and electron conductivity (MIECs), such as cobalt-based perovskites. For example, the oxygen self-diffusion coefficient (D*) and the oxygen surface exchange coefficient (k) for LSM20 at 800 °C were found to be 4.00 × 10
−15 cm
2 s
−1 and 5.62 × 10
−9 cm s
−1, respectively, compared to D* = 9.87 × 10
−10 cm
2 s
−1 and k = 6.31 × 10
−7 cm s
−1 for the La
0.8Sr
0.2CoO
3−δ MIEC material [
72]. The values of the ionic conductivity, σ
i, for LSM are in a range of 10
−4–10
−7 S cm
−1 at 800–1000 °C and decrease significantly in the intermediate temperature range of 600–800 °C [
30].
Recently Jiang summarized the literature data regarding the characterization of the oxide materials from the lanthanum strontium cobaltite ferrite (La,Sr)(Co,Fe)O
3−δ series, and showed that the composition La
0.6Sr
0.4Co
0.2Fe
0.8O
3−δ (the acronym LSCF will be used from now on), as a representative of MIECs, is the most prominent cathode material used in IT-SOFCs [
33]. LSCF, which has a perovskite structure with rhombohedral distortions [
73,
74], exhibits excellent electrical properties properties with ionic and electron partial conductivity values reaching approximately 1 × 10
−2 S cm
−1 and 1 × 10
2 S cm
−1, respectively, at 800 °C [
75]. It demonstrates a moderate CTE value equal to 17.5 × 10
−6 K
−1 in the range of 30–1000 °C [
76]. The oxygen self-diffusion and surface exchange coefficients for LSFC amount 5 × 10
−7 cm
2 S
−1 [
77] and 6 × 10
−6 cm S
−1 [
78] at 800 °C. It should be noted that due to MIEC conductivity nature and the superior oxygen diffusion properties of LSCF compared to LSM, this material is preferred for use in electrochemical devices operated at lower temperatures, whereas LSM materials are still in high demand for high-temperature applications due to their CTE values being more compatible with electrolyte materials and higher total conductivity values.
The main drawbacks of conventional perovskite electrodes, which lead to the degradation of solid oxide cells during long-term operation, are the segregation of Sr at the electrode surface with the formation of the SrO layer for the LSM- [
35,
52] and LSCF-based [
36,
79,
80,
81,
82] cells, and the high interaction of LSM [
41,
52,
83,
84,
85,
86] and LSCF [
41,
87,
88,
89,
90] with Zr-containing electrolytes to form the SrZrO
3 (SZO) and La
2Zr
2O
7 (LZO) phases. In addition, due to the presence of CO
2 in the air, the SrCO
3 carbonate phase was formed on the surfaces of the LSM- [
91,
92,
93] and LSCF- [
94,
95,
96] electrodes. The formation of the same insulating phases limits the oxygen exchange at the electrode–electrolyte interface and reduces the electrocatalytic activity of the LSM [
97,
98,
99,
100] and LSCF [
79,
100,
101,
102,
103,
104,
105] air electrodes for the ORR, followed by an increase in both the electrode ohmic and polarization resistances. Similar long-term operation tests have shown that the degradation of cells based on LSM [
81] or LSCF [
106,
107] operating in electrolysis mode was higher than that in fuel cell mode, and when comparing two perovskite electrodes, the long-term durability of LSCF was one step ahead of that of LSM [
106,
108].
Automated methods developed for the detection of Sr nucleation seeds on the electrode surface [
109,
110], for the identification of the interlayer width between the perovskite and YSZ layers [
111,
112], and for the computational design and numerical simulation of the perovskite-based composite electrodes [
113,
114,
115,
116] allowed for the suggestion of possible degradation mechanisms and an understanding of the electrode behavior at both the atomic and macroscopic scales. The deposition of protective and non-catalytic layers on the electrode surface has been proposed as a solution to the problems of segregation [
117,
118,
119], contaminant poisoning [
120,
121,
122,
123], and sluggish oxygen kinetics [
124,
125,
126,
127,
128]. The organization of ceria-based buffer layers at the perovskite electrode/YSZ electrolyte interface and the replacement of the zirconium electrolyte in the composite electrode with other ionic conductors, methods widely used for other perovskite electrodes [
129,
130,
131,
132,
133,
134,
135], may also help to reduce interactions and increase the activity and long-term stability of LSM [
136,
137,
138] and LSCF [
138,
139,
140,
141,
142,
143,
144,
145,
146,
147] electrodes. The high reactivity of the (La, Sr)-containing electrodes with the conventional YSZ electrolyte facilitated extensive investigations of the LSM-based cathodes formed on the SDC [
148,
149,
150,
151], GDC [
152], SSZ [
151,
153], LSGM [
149], BaZr
0.8Y
0.2O
3−δ (BZY20) [
154], La
9.5Si
6O
26.25 [
155], and La
27.44W
4.56O
55.68 (LWO56) [
156] electrolytes as an alternative. The LSCF-based cathodes were formed on the SSZ [
157], SDC [
158,
159], Ce
0.9Gd
0.1O
2−δ (GDC10) [
160,
161,
162], GDC [
126,
163,
164,
165,
166,
167] Y
0.1Ce
0.9O
1.95 [
168], Nd
0.2Ce
0.8O
3−δ [
169], SSZ [
170], LSGM [
171,
172], GDC with LSGM [
173], SDC with LSGM [
174], BZY20 [
175], BaZr
0.9Y
0.1O
3−δ [
176], BaZr
0.8Yb
0.2O
3−δ (BZYb) [
177], BaCe
0.7Zr
0.1Y
0.1Yb
0.1O
3−δ (BCZYYb) [
178,
179,
180,
181,
182], BaCe
0.7Zr
0.1Y
0.2O
3−δ (BCZY) [
183,
184,
185], and BaCe
0.7Zr
0.15Y
0.15O
3−δ (BCZY15) [
186] electrolytes.
The chemical compatibility of the LSM and LSCF electrodes with oxygen-ion and proton-conducting electrolytes and the interdiffusion across the cathode/interlayer/electrolyte interfaces have been extensively observed in the recent reviews of Zhang et al. [
41], Khan et al. [
42] and Hanif et al. [
187]. To briefly summarize the data presented in [
41], it could be mentioned that LSM20, in contrast to LSCF, showed good chemical compatibility with La
27W
4NbO
55−δ up to 1400 °C [
188] and with La
10Si
55Al
0.5O
26.75 at 1300 °C [
189], whereas the La-deficient LSM40 did not react with La
0.9Sr
0.1Ga
0.8Mg
0.2O
2.85 (LSGM9182) at 1300 °C [
190]. Secondary phases were found to form after sintering of LSM20 with SDC and BaCe
0.9Y
0.1O
3−δ at 1150 °C [
191], La-deficient LSM20 with BZY20 and BaCe
0.8Y
0.2O
3−δ (BCY20) at 1100 °C [
192], LSCF with BZY20 and BCY20 at 1100 °C [
192]. The conclusions in [
41] justified that LSCF had better chemical compatibility with LSGM and CeO
2-based electrolytes due to the formation of small amounts of impurity phases with LSGM9182 at 1300 °C and negligible interdiffusion with SDC at 1150 °C.
It should be noted that the use of LSM electrodes in contact with the BaCeO
3-based electrolytes is limited due to their active chemical interaction [
193]. However, due to its high electronic conductivity and CTE compatibility, LSM can be successfully used as a collector for perspective layer electrodes for proton-conducting fuel cells operating in the IT range [
194,
195,
196,
197].
This entry is adapted from the peer-reviewed paper 10.3390/ma16144967