Effective control of blood loss is able to save time and improve the survival rate of patients. When bleeding occurs, hemostasis is the body’s spontaneous response. The mechanism of hemostasis in vivo involves two processes: primary hemostasis, when endothelium gets injured, and collagen and other subendothelial matrix components are exposed, and von Willebrand factor is released to allow platelets to adhere to the wound site; secondary hemostasis, tissue factor stimulates the conversion of prothrombin to thrombin, and soluble fibrinogen acts to limit the formation of insoluble fibrin clots. Nanotechnology can transform and utilize the microstructure on the nanoscale, which gives nanomaterials unique advantages such as improved diffusivity and solubility, easy-to-penetrate physiological barriers, large specific surface area, slow control, and targeted release of drugs.
1. Nanoparticles
Nanoparticles refer to colloidal particles composed of macromolecular substances with a solid particle size of 10–1000 nm. Charged nanoparticles can produce an electrostatic effect with blood cells or fibrinogen with opposite charge, neutralize the surface charge, induce its aggregation, and promote blood coagulation [
41]. Blood is an important medium to help nanoparticles reach target tissues and organs. Moreover, they also have unique flow influence characteristics, which can affect the distribution of platelets and nanoparticles in the vascular system [
42,
43]. Additionally, nanoparticles can easily penetrate biological barriers, enter systemic circulation, and penetrate cells via endocytic processes, including pinocytosis, phagocytosis, or endocytosis [
44]. Nanoparticles cause changes in erythrocytes, which affect the viscosity of blood and thus play the role of hemostasis. Zheng et al. encapsulated bovine serum albumin (BSA) and chitosan (CS) in a mesoporous bioactive glass (MBG) nanoparticle (MBG@BSA/CS) that activates physiological coagulation pathways. Significant effects were observed on both surface and internal bleeding in SD rats [
45]. Blood compatibility is one of the main standards for approving all nanomedical devices in contact with blood. Silver nanoparticles (AgNPs) with antibacterial properties and the potential to promote platelet aggregation are one of the more commonly used hemostatic nanomedical materials, which can cause dose-dependent hemolysis. Researchers used sodium bis (2-ethylhexyl) sulfosuccinate (AOT), polyvinylpyrrolidone (PVP) Silver nanoparticles coated with polylysine (PLL) and bovine serum albumin (BSA) delayed plasma coagulation. Only PLL-type AgNPs could inhibit plasma coagulation and induce platelet activation, thereby interfering with hemostasis [
46]. Red blood cells and platelets are key participants in the coagulation process. They promote coagulation through the procoagulant activity (PCA) complex expressed on their surface. Blood coagulation is a complex process that involves not only these cells in the blood but also biochemical components such as coagulation factors. The coordinated interaction of these key cells and biochemical components is necessary to maintain hemostasis and prevent excessive bleeding. Causing platelet aggregation, interfering with plasma coagulation, and inducing the production of leukocytes, PCA can be used to measure the possibility of nanomaterials promoting coagulation or anticoagulation in vivo and in vitro. In vitro test shows that the adjustment of nanoparticle concentration can effectively regulate platelet aggregation, interfere with plasma coagulation and activate leukocyte PCA [
47]. However, most studies focus on the function of nanoparticles and ignore the potential risk that nanoparticles may disrupt the hemostatic balance and may also lead to thrombosis and bleeding [
48].
2. Nanosheets
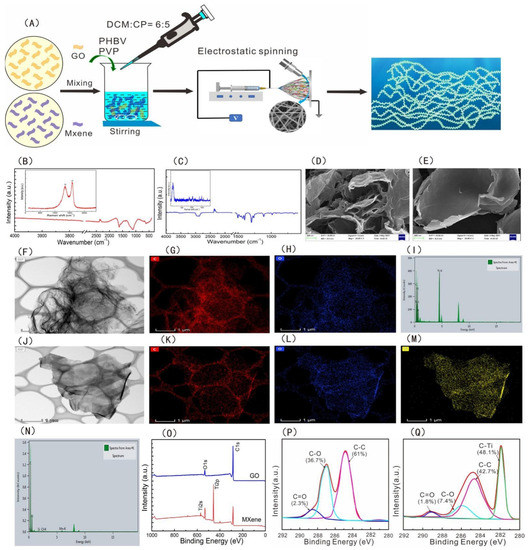
Freestanding super thin film, often referred to as nanofilm or nanosheet, is a new two-dimensional nanomaterial that has attracted much attention in the field of nanotechnology in recent years. Nanosheets have a large surface area/aspect ratio with high transparency and superior flexibility. When applied to wounds, platelets, blood cells, and coagulation factors can be concentrated through rapid water absorption. The negative charge on its surface can activate the endogenous coagulation pathway, so as to achieve the purpose of hemostasis. For example, Wu et al. prepared a novel nanofilm with high antibacterial hemostatic ability by embedding GO/MXene nanosheets uniformly on the surface of PHBV fibers. Compared with the PHBV membrane, the tensile strength, platelet adsorption, and clotting time of the membrane with nanosheets were significantly increased, and the antibacterial rate of the membrane could reach 97% (see
Figure 3 below) [
49]. Shi et al. combined montmorillonite nanosheets with Lycium barbarum polysaccharide to prepare hemostatic hydrogel, which had a three-dimensional porous structure and hydrophilic surface, which was conducive to the rapid adsorption of blood. In addition, in vivo experiments have proved that it can effectively reduce the tissue damage caused by inflammation and shorten the wound healing time, which has great application potential in the clinical treatment of hemostasis and wound healing [
50]. Xuan et al. developed a GDP@Ca
2+/PCL nanosheet, which has high strength, can stop bleeding and be antibacterial, and can adhere to uneven tissues in vivo environments. In addition, by combining Ca
2+ and antimicrobial peptides, the nanosheet demonstrated excellent hemostatic and anti-infective properties in mouse models of back skin and liver damage [
51]. However, the mechanism of the nanosheet on the coagulation process is not clear, and the interaction between the material and platelets and blood cells, as well as the stimulation of the endogenous coagulation pathway, need to be further studied.
Figure 3. (
A) Schematic diagram of preparation of the PHBV−GO/MXene composite membranes. FT−IR and Raman spectra of (
B) GO and (
C) MXene. SEM images of (
D) GO and (
E) MXene. TEM analysis of (
F) GO and (
J) MXene. Distribution of elements: (
G) C for GO, (
H) O for GO, (
K) C for MXene, (
L) O for MXene, and (
M) Ti for MXene. The element composition of (
I) GO and (
N) MXene. (
O) XPS spectra of GO and MXene. C1s XPS spectra of (
P) GO and (
Q) MXene [
49]. Copyright © 2021 by the authors. Licensee MDPI, Basel, Switzerland.
3. Liposomes
Liposomes usually refer to spherical bilayer lipid molecules with a diameter of 20 nm to 10 μm made of phospholipids and cholesterol. The properties, types, surface charges, particle sizes, and preparation methods of liposomes vary widely. It is a widely studied nano-delivery system [
52]. Liposomes can encapsulate coagulation factors, prevent coagulation factors from being cleared and inactivated, and improve the circulation time of coagulation factors in vivo. Liposomes can also be coupled with the hemostatic polypeptide chain to enhance the stability of the polypeptide chain while exerting its hemostatic effect [
53]. Modery-Pawlowski et al. modified liposomes with collagen and VWF-binding peptides, CBP and VBP, to promote platelet aggregation. This liposome construct has shown significant hemostatic effects in a mouse tail amputation model [
54]. In addition, researchers enclose thrombin in liposomes and deliver it to isolated platelets, which will allow transfusion platelets to coagulate more easily in response to platelet agonists and eliminate the increased risk of thrombosis caused by thrombin delivery [
55]. Furthermore, nanoparticles have been applied to deliver components that activate coagulation factors, and combined liposomes with nanoparticles to prepare new hemostatic nanomaterials. For example, Donovan et al. encapsulated polyphosphate nanoparticles into liposomes, and this nanomedicine can shorten the clotting time of plasma and show good procoagulant properties [
56].
4. Nanofibers
Nanofiber refers to a linear material with a certain length diameter ratio with a diameter of nanoscale and a large length. In a narrow sense, the diameter of nanofiber is between 1 nm and 1000 nm. Nanofiber has the characteristics of surface effect, easy combination with other atoms, small size effect, such as melting point reduction, color separation, and discoloration, quantum size effect, macro quantum positive tunnel effect, etc. Nanofibers can mimic the nanomorphology characteristics of fibrin fibers during natural hemostasis, and have a large surface area, adjustable porous structure, and precisely controllable structure; hence nanofibers have a strong potential for hemostasis [
57,
58]. Xianrui Xie and others prepared an ultra-light 3D gelatin sponge composed of nanofibers. In vitro evaluation showed that the sponge had good cell compatibility, high cell permeability, and low hemolysis rate. Subcutaneous implantation studies in rats have shown that the sponge aggregates and activates large numbers of platelets accelerates embolism formation, and promotes other coagulation pathways to accelerate clotting. The in vivo study of the rabbit ear artery injury model and liver injury model shows that compared with commercial gelatin hemostatic sponge, gelatin nanofiber sponge can quickly induce stable thrombosis and perform the least blood loss. The above findings suggest that gelatin nanofiber sponge is a potential absorbable hemostatic agent [
59]. Shixuan Chen et al. have developed an injectable and super elastic nanofiber matrix. Compared with commercialized hemostasis materials, the matrix exhibits greater absorption/blood capacity and has high efficiency in whole blood coagulation assay, especially for thrombin-immobilized samples. Further in vivo tests showed that the nanofiber matrix was effective in hemostasis in the pig liver injury model [
60]. Li et al. used carbon nanofibers (CNFs) to promote fibrin growth and cause rapid clotting, with super fiber hydrophobicity limiting blood wetting to prevent blood loss and antibacterial. In addition, after the clot shrinks, it can be peeled off automatically, avoiding tissue damage. The above characteristics of the material make it a good application in the field of hemostasis [
29]. Other researchers expanded the two-dimensional (2D) nanofiber membrane to three-dimensional space to obtain a three-dimensional (3D) layered nanofiber sponge, which increased the interface interaction between sponge and blood cells and accelerated hemostasis. The structural adjustment of this nanofiber shows good elasticity, high permeability and liquid absorption rate, and high compressibility and elasticity, which is conducive to filling the deep wound and promoting tissue healing. Moreover, the 3D dynamic environment can regulate the tissue cells of the wound and promote the regeneration of the dermis and the recovery of cells. Full-thickness skin defect experiments in mice showed that 3D layered nanofibers effectively accelerated wound healing and reduced scar formation [
61].
5. Self-Assembling Peptides
The ordered nanostructured peptides formed by relatively simple peptide chains through noncovalent self-assembling nanopeptides are able to form a nanofiber barrier in any humid ionic environment in the body and concentrate blood components to control bleeding. It can be decomposed into natural amino acids in vivo with good biocompatibility. Min Wu et al. synthesized a hemostatic agent by solid-phase synthesis, which is a bifunctional, biodegradable, self-assembling nanopeptide (SAP) ADA16-I. The (SAP) ADA16-I solution will self-assemble into a barrier to prevent blood flow and promote the movement of adjacent cells to repair the damaged part. In the mouse model of the brain, femoral artery, and liver incision, local treatment with different concentrations of (SAP) rada16-iI solution can significantly shorten the hemostatic time. At the same time, it has the ability to bone repair. When applied to the New Zealand rabbits, it was found that the bone wax in the control group inhibited osteogenesis, while ADA16-I showed effective bone regeneration function in radiological analysis and histological examination with no serious inflammatory reaction [
62]. Kumar et al. assembled a collagen-mimetic peptide (KOD) to form spiral nanofibers that promote thrombosis. KOD can activate platelets and coagulate plasma and blood. In addition to promoting thrombosis, it can also promote the production of pro-inflammatory factors (TNF-α or IL-1β). This novel self-assembling collagen mimetic peptide has good hemostatic properties [
34]. Another research applies the peptide amphiphilic PA self-assembling into nanofibers as the carrier for delivering targeted tissue factor (TF), and chose tissue factor as the target because it was only exposed to the intravascular space when the blood vessel was ruptured and delivered to the damaged site. In the mouse great saphenous vein laser injury model, the self-assembling nanofibers reduced the blood loss by 35% to 59%; TF-targeted nanofibers can selectively locate the injury and TF exposure sites while reducing blood loss [
63]. Another study developed an intravenous targeted tissue factor (TF) nano-therapy to stop bleeding. Three tissue factor-specific binding peptides were covalently bound to the backbone of the PA peptide chain and self-assembling into three kinds of nanopeptide fibers. All nanofibers were able to bind to liver bleeding sites, but only RTL nanofibers reduced blood loss by 53% compared with sham surgery, and increasing the density of targeted ligands of RTL nanofibers resulted in better binding to injury sites and reduced blood loss in vivo. Peptides successfully bound TF in vitro and successfully bound TF-targeted PA nanofibers to bleeding sites, thereby reducing blood loss in vivo [
64].
6. Nanocomposite Hydrogel
Gels are high molecular polymers with a three-dimensional network structure that can absorb large amounts of water or biological fluids. When the polymer structure of the gel contains hydrophilic groups, the gel will be more absorbent. The gel can swell in water without dissolving due to its three—dimensional crosslinking structure [
65]. However, low mechanical strength limits the application of the gel. In recent years, nanocomposite hydrogels have become a new type of biomaterial due to the improvement of shear thinning properties [
66]. The properties of hydrogels can be enhanced by adding a variety of nanomaterials as nano-fillers into the soft polymer matrix, so as to form nanocomposite hydrogels with improved properties. Nanocomposite hydrogels not only have high hydrophilicity, strong water absorption, and flexibility, but also have large specific surface area and strong adsorption capacity. Nanocomposite hydrogels can concentrate blood cells and platelets, and help to stop bleeding. At the same time, a layer of gel film is formed on the surface of the wound to block the wound. Many acute injuries result in irregular wounds or intracavitary bleeding, preventing thin films or sheets of hemostatic material from penetrating deep into the wound to being effective. Nanocomposite gels, on the other hand, come in powder form and form a gel in situ, which can be used for hemostasis in irregular and deep wounds. These physical properties make gels more similar to living tissues, so gels are gradually used as alternative materials for biological tissues [
67]. Sundaram et al. combined nano-bioglass (nBG) with silica, calcium, and phosphate ions into chitosan (Ch) hydrogel as a hemostatic agent. In the analysis of the hemostatic effect in vitro and in vivo, compared with control 2% Ch hydrogel, 2% Ch- 5% nBG hydrogel formed rapid blood clots, which indicates great potential to effectively stop bleeding of nanocomposite gels [
25]. Chen et al. prepared a composite hydrogel by mixing pectin and cellulose. This composite hydrogel has a dense structure while retaining the crystal structure of cellulose I and II and good thermal stability. In vivo experimental results showed that liver wounds treated with hydrogel reduced bleeding within 3 min, further highlighting the potential of the composite hydrogel as a biomedical material for rapid hemostasis [
68]. Zhao et al. combined the advantages of biomacromolecules and clay to prepare a hydrogel dressing with Eloxite nanotubes and chitin as the main components. Au@HNTs-Chitin composite hydrogel has high antibacterial and hemostatic activity with low cytotoxicity, which shows the function of promoting wound healing. This research shows broad application prospects in wound antiseptic hemostasis [
69].
This entry is adapted from the peer-reviewed paper 10.3390/molecules28135264