Metallogels are a category of materials formed by combining polymer gels with metal ions, creating coordination bonds with the functional groups of the gel. The incorporation of metal phases into hydrogels offers diverse possibilities for functionalization. Cellulose stands out as a preferred choice for producing hydrogels from various standpoints, including economic, ecological, physical, chemical, and biological aspects. It possesses advantages such as cost-effectiveness, renewability, versatility, non-toxicity, remarkable mechanical and thermal stability, a porous structure, a significant number of reactive OH groups, and excellent biocompatibility.
1. Introduction
Naturally derived gels are a novel class of materials with unique physical and chemical properties that make them versatile for a wide range of applications [
1].
Generally, hydrogels can be prepared from synthetic or bio-based polymers. Synthetic polymers are the products of petroleum, while bio-based polymers have natural sources—they are derived from plants and living organisms [
2,
3,
4,
5]. Hydrogels from synthetic polymers are less hydrophilic and mechanically more robust compared to bio-based hydrogels [
6]. However, the latter are far more preferable due to their unique advantages, such as non-toxicity, biodegradability, low cost, renewability and abundance of the sources [
1,
7,
8,
9].
Cellulose, starch, chitin, and chitosan are examples of natural-based biopolymers with hydrophilic functional groups that can absorb and retain a large amount of water [
7,
8,
9,
10,
11,
12]. Cellulose is a pristine material for several thousand types of useful products for the chemical industry, including oligo- and monosaccharides and their derivatives, fibers, films and other materials [
8,
13,
14]. Cellulose is preferable for the production of hydrogels and their further functionalization from economical, ecological, physical, chemical, and biological points of view since it is inexpensive, renewable, versatile, non-toxic, reveals high mechanical and thermal stability, has a porous structure, an imposing number of reactive OH groups, and good biocompatibility.
Hydrogels are mostly produced from nanocellulose [
15,
16] and bacterial cellulose [
17,
18], both are not advantageous at an industrial scale, while plant-derived cellulose in the current state of the art has comparatively less often been applied in the preparation of hydrogels because of its poor solubility. This drawback can be overcome by obtaining cellulose derivatives via various chemical modification procedures, such as esterification, etherification, or oxidation [
7,
15,
19,
20,
21,
22]. However, this means additional steps of derivatization and different properties of the derivatives because of the introduction of the new functional groups.
Non-derivatized cellulose hydrogel can be obtained from a cellulose solution through physical cross-linking by hydrogen bonding with hydroxyl groups [
23]. In this case, the three-dimensional polymer structure captures the solvent molecules in the framework cavities due to various supramolecular interactions, namely hydrogen bonding, π–π stacking, or van der Waals interactions [
24]. Additionally, a number of cellulose hydrogels preparation methods include chemical cross-linking in order to improve the properties of the hydrogel. Epichlorohydrin (ECH), aldehydes and aldehyde-based reagents, urea derivatives, carbodiimides and multifunctional carboxylic acids are the most widely used crosslinkers for cellulose [
25].
Cellulose-based hydrogels give rise to a huge number of composite materials. They possess three-dimensional porous structures, which allow the incorporation of functional fillers and are thus applied as smart materials [
16,
22,
26,
27,
28,
29,
30]. The same, the use of cellulose aerogels is preferable to obtain special properties with new potential applications [
27,
28,
29,
30,
31]. Nanocomposites based on cellulose hydrogels with metal particles have been extensively developed in recent decades. In such compounds, cellulose acts as a matrix for metallic and metal oxides nanostructures. These metal/cellulose composites could be synthesized via chemical (atomic or molecular condensation, sol-gel process, chemical vapor deposition, colloidal method), physical (ultrasonication, laser pyrolysis or ablation, spluttering and others) or biological organisms (e.g., bacteria, fungi, viruses, algae, plants act as reducing or stabilizing agents) routes [
31].
The most desirable for biomedical applications are small nanoparticles (less than 100 nm) uniformly distributed over the bulk of the hydrogel [
29]. Although for some purposes, e.g., wound dressings, the presence of nanoparticles only in the surface layers may be sufficient. Therefore, the methods of nanoparticle synthesis are focused on the possibility of managing the size and distribution of the particles due to the selection of special conditions for the method [
32,
33,
34,
35]. Additionally, since metal nanoparticles tend to agglomerate and, as a result, grow and reach bigger sizes (up to dozens of microns), special reagents—stabilizers gain significant importance. The matrix for the introduction of nanoparticles, a cellulose hydrogel, plays an important role too. It traps the particles and holds them due to the physical and chemical bonding performing as the additional stabilizer of growth and prevents washing the particles out. Thus, metal particles, penetrating into the polymer matrix and coordinating with the functional groups of the cellulose hydrogel, form a metallogel in which the metal is a part of the gel network as a coordinated metal ion (in a discrete coordination complex), as a cross-linking metal node with a multitopic ligand (in coordination polymer), and as metal nanoparticles adhered to the gel network [
24]. These materials are called metallogels [
24], or gel-nanocomposites [
36], or metal nanocomposites [
37]. However, the latter is a much broader term, as includes a wide range of materials other than polymer gels.
2. Cellulose Metallogels
At present, composite materials are of great interest. Metal nanoparticles incorporated into the polymer matrix add new properties to the composite, expanding the scope of its application, whereas the amount of metal can be rather small or, on the contrary, it can make up a big share of the composite. The need for control of the particle dispersiveness, their size, and organization on the surface and in the bulk of polymer supports is one of the main tasks.
The first approach to the fabrication of cellulose metallogels is to prepare metal nanoparticles separately and then mix them with the polymer; then cross-linking takes place after mixing the polymer solution and the suspension with nanoparticles [
51,
52]. This method leads to a uniform distribution of nanoparticles in the polymer matrix; however, it is a multistaged procedure, and it involves expensive industrially produced nanoparticles. The second, single-pot and cost-effective approach to obtaining metal nanoparticles is the reduction of metal ions from the aqueous solutions of their salts directly inside the polymer matrix [
53,
54,
55]. This process is known as the diffusion-reduction method and it is available to all types of cellulose matrices, including hydrogels and metallogels. Cellulose suits the production of metallogels due to its extensive pore network. The pores limit the agglomeration of metal nanoparticles and promote their fixation both on the surface and in the inner layers of the cellulose material. It was shown that the structure of cellulose after the introduction and reduction of metal nanoparticles did not change. Therefore, cellulose is considered a neutral nanoreactor [
53]. Hydrogels produced from pristine cellulose along with aerogels, films, powder samples, and fibers were successfully applied as scaffolds (carriers, matrices, sacrificing templates) for intercalation of transition zero-valent d-metals, such as cobalt, nickel, silver, gold, platinum, palladium, iron, copper, zinc or/and their oxides [
51,
52,
53,
54,
55,
56,
57,
58,
59,
60]. For example, it was shown that the hydrate cellulose film could be successfully used to obtain nickel nano- and microspecies located mainly on the surface or in the near-surface layer. A specific feature of nickel species synthesis and embedding into the cellulose film was that the reduction and formation of nickel particles occurred in the solid matrix, which somehow limited the growth of species [
53]. In another study, nanosized cobalt particles were prepared by chemical reduction within a microcrystalline cellulose matrix. Two different chemical reducers, NaBH
4 and NaH
2PO
2, were applied. Particles obtained via the NaBH
4 reduction were amorphous Co-B or CoO composites with diminished ferromagnetic behavior and particles made via the NaH
2PO
2 reduction were well-ordered ferromagnetic cobalt nanocrystals [
55]. The synthesis of the nanoparticles was carried out under heterogeneous conditions which helped to limit the agglomeration of metal particles. The same occurs in fibers or hydrogels, for instance, the silver and gold nanoparticles were embedded into the cellulose matrices in [
56]. Composites cellulose–Au and cellulose–Ag contained a low amount of reduced metals and demonstrated antimicrobial properties. All these examples used the diffusion-reduction method to obtain hybrid cellulose composites with metals. This way may be successfully adapted to the preparation of metallogels via modification of the ready hydrogels.
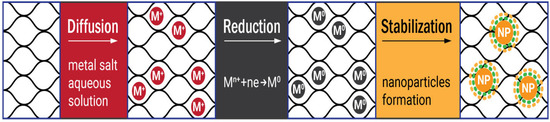
The first step of the diffusion-reduction process is immersing the prepared hydrogel into the aqueous solution of metal salt to provide diffusion of the metal ions into the cellulose matrix. The size distribution of the nanoparticles can be controlled by varying the concentrations of the salts. The reducer is added in the second stage of the process. The reaction results in the colloidal suspension outside the hydrogel, while inside it the metal ions transform to zero-valent metal particles. Finally, the cellulose metallogels are washed with distilled water to remove unfixed metal particles [
54,
56].
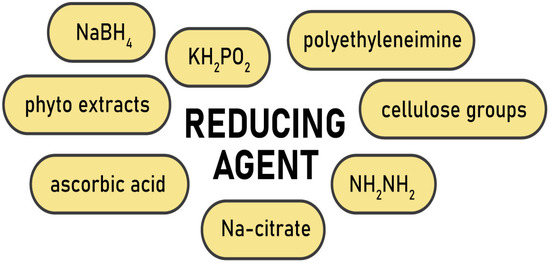
Depending on the type of metal and the desired characteristics of the nanoparticles, different reducing agents are used (
Figure 1). For instance, active sodium tetrahydridoborate or mildly active potassium hypophosphite and hydrazinium hydrogen sulfate are often applied as reducers for zero-valent metals such as Ni, Co, Cu, Fe [
37,
53,
61], and sometimes for Au [
62] and Ag [
63]. Ascorbic acid was employed to prepare cellulose–Ag nanocomposites in the microwave-assisted process [
64], while polyethyleneimine reduced Au
+ from an aqueous suspension [
65]. However, for noble metals a relatively simple and reproducible Turkevich method is preferable. It engages trisodium 2-hydroxypropane-1,2,3-tricarboxylate (trisodium citrate) to reduce silver and gold ions for the synthesis of spherical nanoparticles (
Figure 2) [
54,
56,
66].
Figure 1. Reducing agents for production of metal nanoparticles.
Figure 2. The scheme of the diffusion-reduction Turkevich process in the bulk and on the surface of the hydrogel matrix.
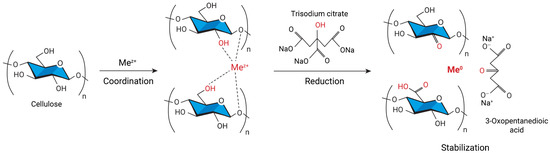
The mechanism of metal reduction by the Turkevich method is shown in
Figure 3. Metal ions diffuse into the cellulose matrix and coordinate with its functional groups. The reducing agent, trisodium citrate, is oxidized in this reaction to 3-oxopentanedioic acid, some of the cellulose OH groups are oxidized to carbonyl or carboxyl groups, and the metal ions are reduced to the zero-valent state. The metal particles grow and form a nanoparticle, which is stabilized by cellulose, 3-oxopentanedioic acid, and trisodium citrate. Stabilization of the particles prevents further agglomeration, which makes it possible to obtain smaller nanoparticles [
35,
56,
67]. The metal reduction depth depends on the aspect ratio between cellulose and metal ions. The less the aspect ratio, the less the share of cellulose and, respectively, higher the amount of zero-valent metal in the solutions [
56].
Figure 3. The mechanism of the redox reaction leading to the formation of metal nanoparticles in the cellulose matrix.
Strange as it may sound, the reduction step may be carried out without reducers due to the functional groups and bounds of the cellulose matrix of the hydrogel. In this case, it is usually called “green synthesis”. Cellulose contains the end aldehyde groups; therefore, the reaction occurs without the addition of chemical reducers. For example, Au(0) nanoparticles were synthesized in a cellulose hydrogel without a reducer in [
56]. However, cellulose has a rather moderate number of end aldehyde groups, not enough to interact with a big number of metal ions. As a result, only low content of metal nanoparticles may be obtained in the composite hydrogels. Cellulose derivatives are able to perform the reduction of metal ions as well. For instance, Ag nanoparticles were obtained in a carboxymethyl cellulose (CMC) hydrogel, where the reducing agent was CMC [
68]. Not only cellulose functional groups but also cross-linking bounds in the polymer can provide reduction, for instance, C = N bonds in the hydrogel prepared from amino cellulose and dialdehyde xylan [
16].
Another “green” option for obtaining metal nanoparticles by reduction from their salts but without chemicals is a phyto-synthetic route which is related to the use of aqueous plant extracts. Plant parts (leaf, stem, root, fruit, and seed) produce phyto-chemicals naturally rich in amino, carboxyl and hydroxyl groups for triggering the formation of metal nanoparticles, as well as stabilizing and/or capping them [
69]. Phyto-synthesis has a good scaling potential for the production of stable, varied in shape and size nanoparticles of metals and metal oxides. Currently, this biosynthetic way is commonly used for the production of ZnO nanoparticles from plants such
as Ferulago angulata,
Bergenia ciliata,
Sageretia thea,
Anisochilus carnosus,
Limonia acidissima L., and
Syzygium cumini [
69,
70,
71,
72,
73,
74].
Metals can also initiate the gelation process and act as cross-linking agents during the formation of metallogels. The ability of transition monovalent metal ions to start the gelation of nanofibrillated cellulose (NFC) was revealed by Dong and his research group. During the TEMPO process that was applied for NFC production, negatively charged surface carboxylate groups that provide high binding capability to transition metal species (e.g., Ag
+) were generated. Ag
+ was bound on the NFC surface and simultaneously induced the formation of NFC-Ag
+ hydrogels. Subsequently, Ag
+ ions were slowly reduced to Ag(0) nanoparticles by hydroxyl groups on NFC without an additional reducing agent. Thus, the stiff NFC-Ag
+ hydrogel was initiated by strong association of carboxylate groups on NFC with Ag
+ and sufficient NFC surface charge reduction [
75]. Zander and co-workers reported novel nanocellulose hydrogels fabricated via hydrogelation using metal salts. In this method, Ca
2+ and Fe
3+ played the role of cross-linkers [
76].
In contrast to metallic nanostructures that are mainly limited to noble or semi-noble metals, practically any type of metal oxide can be deposited on the cellulose surface with TiO
2, Fe
3O
4, and ZnO being the most abundant. For this purpose, various cellulose types have been applied, spanning from natural cellulose fibers to cellulose nanocrystals in the original or chemically modified form [
48].
Summing up, we can highlight two main chemical methods for the preparation of cellulose-based metallogels. The first one is a modification of the diffusion-reduction method, when the hydrogels prepared in advance are immersed in a solution of a salt, and then the metal ions react with chemical reducing agents resulting in the metal nanoparticles. Additionally, the reduction may be carried out due to the reducing ability of cellulose itself or phyto-chemicals. This method can be utilized to obtain metallogels with almost any transition metal, among which silver is the most popular. The second method involves the direct participation of metal ions in gelation or cross-linking after mixing a solution of salt with a cellulose solution.
This entry is adapted from the peer-reviewed paper 10.3390/gels9050390