Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Environmental Sciences
Compared to diesel, liquefied natural gas (LNG), often used as an alternative fuel for marine engines, comes with significant advantages in reducing emissions of particulate matter (PM), SOx, CO2, and other pollutants. Promoting the use of LNG is of great significance for achieving carbon peaking and neutrality worldwide, as well as improving the energy structure.
- LNG engines
- CH4 emission
- NOx emission
- nonthermal plasma
1. Introduction
Ship transportation undertakes over 90% of global transportation tasks [1]. Marine engines using traditional petroleum or heavy oil as fuel can cause environmental damage and air pollution, such as nitrogen oxides (NOx), SOx, particulate matter (PM), etc. [2]. Choosing alternative fuels (such as methanol, bioethanol, natural gas, etc.) is an effective way to reduce the exhaust pollution of marine engines [2,3,4]. As an alternative fuel for marine engines, liquefied natural gas (LNG, mainly composed of CH4) has shown significant advantages in reducing emissions such as PM and SOx [5,6]. At the same time, LNG has a lower C/H ratio, which can reduce CO2 emissions by about 30% compared to diesel [7]. Promoting the use of LNG is of great significance for achieving carbon peak and carbon neutrality worldwide and is also important for improving the energy structure.
However, compared to diesel engines, medium- and high-speed marine LNG engines may produce higher methane (CH4) emissions and also have NOx emission issues [8,9]. CH4 is a kind of greenhouse gas, and its halocarbon global warming potential (HGWP) is about 28 times that of CO2 [10]. NOx is one of the important factors that cause harmful weather, such as photochemical smog, haze, and acid rain. Long-term inhalation of NOx can cause serious damage to the functions of human viscera and even threaten human life. The CH4 and NOx emissions from several types of high-speed marine LNG engines developed by Guangxi Yuchai Machinery Group Co., Ltd. (Guangxi, China) were tested and collated, as shown in Table 1.
Table 1. Emissions from high-speed marine LNG engines (manufactured in 2020).
| No. | kW–r/min | NOx(g/kWh) | NMHC * (g/kWh) | CH4 (g/kWh) | IMO Tier III for NOx (g/kWh) |
|---|---|---|---|---|---|
| 1 | 120–1500 | 2.304 | 0.425 | 4.427 | 2.08 |
| 2 | 138–1500 | 2.085 | 0.401 | 4.186 | 2.08 |
| 3 | 180–1500 | 2.352 | 0.508 | 4.494 | 2.08 |
| 4 | 210–1500 | 1.557 | 0.383 | 3.224 | 2.08 |
| 5 | 265–1500 | 1.703 | 0.279 | 2.695 | 2.08 |
| 6 | 400–1500 | 2.09 | 0.531 | 4.959 | 2.08 |
* NMHC is non-methane hydrocarbon emission.
In order to control the CH4 and NOx emissions from marine LNG engine exhaust, China has issued the “Limits and measurement methods for exhaust pollutants from marine engines (CHINA I, II)” [11], in which the CHINA II stage requires a CH4 emission limit of 1.0 to 2.0 g/kWh. IMO Tier III requires that the NOx emissions of medium- and high-speed marine engines (1000–2000 r/min) should be limited to 2.26–1.96 g/kWh. Moreover, the Euro VI imposes stricter requirements on CH4 and NOx emissions from heavy-duty natural gas engines [12], which means that there is a possibility that the emission standards for ship engines will become increasingly strict. Considering the increasingly stringent constraints on pollutant emissions in the future, it is necessary to deal with CH4 and NOx emissions from marine LNG engines.
Due to the lean combustion strategy, medium- and high-speed marine LNG engines may have a lower exhaust temperature. And the oxygen-enriched condition of marine-engine exhaust limits the use of three-way catalysis (TWC) [13]. For the removal of CH4 and NOx emissions from the exhaust gas of medium- and high-speed marine LNG engines, the traditional technical route of combining a methane oxidation catalyst (MOC) and an HN3 selective catalytic reduction system (NH3-SCR) will lead to the following problems: (1) A large number of noble metal catalysts, such as platinum, palladium, and rhodium, are used in MOC, resulting in high economic costs; and (2) The low exhaust temperature will lead to the low catalytic oxidation efficiency of CH4 [14], and this problem will be exacerbated under low load conditions. Moreover, MOC combined with the SCR system has a large mass and dimensions, which is difficult to install on ships with limited space. And the use of pure ammonia on marine vessels is hazardous and requires special attention.
Some studies have shown that CH4 in the exhaust of LNG engines can be used as a reducing agent to remove NOx from the exhaust gas. Therefore, selective catalytic reduction using CH4 as a reducing agent (CH4-SCR) has been studied as a desirable technology for the removal of NOx and CH4 simultaneously in marine LNG engine exhaust [15]. Compared to the MOC+NH3-SCR technology route, the CH4-SCR system has the significant advantages of low cost, a small footprint, and a simple system [16]. However, due to the high stability of CH4 molecules and the poor low-temperature activity of the CH4-SCR system, its application in the integrated treatment of CH4 and NOx from marine LNG engine exhaust is limited [17]. To improve the low-temperature activity of catalytic systems, non-thermal plasma (NTP) technology was introduced into the traditional catalytic field [18]. The electron temperature in non-thermal plasma is very high (in the order of 104–105 K); the temperature of heavy particles (ions and neutrals) is around 300–1000 K, and the whole system presents a low-temperature state, so it is also called cold plasma, or non-equilibrium plasma. The generation of NTP requires additional energy consumption, which is the key factor affecting the commercial application of the NTP+CH4-SCR system [19]. And optimizing system energy consumption and improving system conversion efficiency requires us to fully understand the synergistic mechanism between NTP and CH4-SCR catalysts.
2. The Introduction of NTP Technology in the CH4-SCR System
For reactions that require high activation energy, the average energy of high-energy electrons generated in NTP is 1 to 10 eV. The high-energy electrons can activate and dissociate gas molecules through inelastic collisions, making many reactions occur that usually require extremely harsh reaction conditions [20]. According to the arrangement of the plasma and catalyst, NTP synergistic catalytic reactors can be divided into two types: post-plasma catalyst (PPC) and in-plasma catalyst (IPC), as shown in Figure 1.
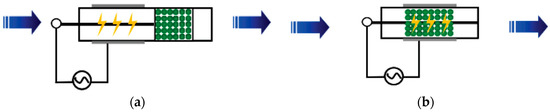
Figure 1. Two types of NTP synergistic catalytic reactors: (a) PPC Reactor and (b) IPC Reactor.
Due to the simultaneous occurrence and interaction of the plasma and catalytic processes in the IPC reactor, the reaction process is more complex and efficient than the reaction in the PPC reactor [20]. In the IPC reactor, the catalyst is arranged in the plasma discharge space, and the pores inside the catalyst will generate plasma in the form of a micro discharge, improving the density of the plasma. The short-lived active species generated by a plasma discharge (such as excited molecules and radicals) can more effectively act on the catalyst and improve the treatment efficiency of the catalyst [21,22]. Figure 2 shows the interaction mechanism between plasma and a catalyst in the IPC reactor.
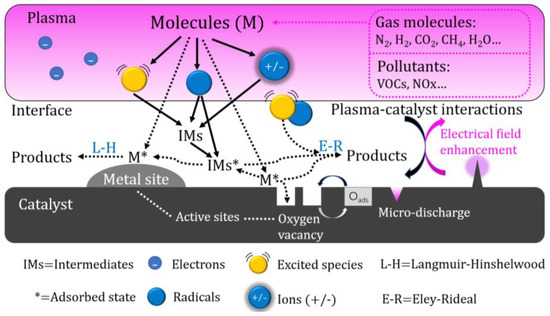
Figure 2. The Synergistic Mechanism of Plasma and a Catalyst in IPC Reactor.
Previous research has demonstrated the significant effect of NTP in producing active species and reducing reaction temperature. CH4 oxidation and NOx reduction are complementary processes, and there are relevant studies on CH4-SCR systems aimed at NOx removal [23,24]. Therefore, adopting the synergetic method of NTP and catalysts (NTP-CH4-SCR) to achieve the integrated treatment of CH4 and NOx from the exhaust of marine LNG engines has become a research hotspot.
On the one hand, the introduction of NTP technology can reduce the reaction temperature of CH4 and NOx removal to overcome the low-temperature limitations of marine LNG engine exhaust. On the other hand, compared to MOC+NH3-SCR, NTP and catalyst synergism to treat CH4 and NOx using only one catalyst can reduce the use of noble metal catalysts, which is of great significance in improving the economy of exhaust after-treatment devices.
Therefore, using the method of synergic NTP and catalyst to achieve the simultaneous removal of CH4 and NOx from marine LNG engines is an effective way to overcome the low exhaust temperature and reduce the use of noble metal catalysts. However, in practical applications, the energy consumption of the NTP synergic catalyst system must be considered, which is related to the system economy. This requires careful consideration of the optimal design of NTP reactors and catalysts suitable for plasma environments [22]. Fully understanding the synergistic mechanism of NTP and catalysts is a prerequisite. At present, the synergistic mechanism between NTP and catalysts is still unclear, which limits the optimization and promotion of energy consumption of the NTP-CH4-SCR system.
This entry is adapted from the peer-reviewed paper 10.3390/ma16144969
This entry is offline, you can click here to edit this entry!
