Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Middle-aged adults can start to be affected by some arterial diseases (ADs), such as abdominal aortic or popliteal artery aneurysms, lower extremity arterial disease, internal carotid, or renal artery or subclavian artery stenosis. These vasculopathies are often asymptomatic or paucisymptomatic before manifesting themselves with dramatic complications. Therefore, early detection of ADs is fundamental to reduce the risk of major adverse cardiovascular and limb events. Furthermore, ADs carry a high correlation with silent coronary artery disease (CAD).
- diagnosis
- secondary prevention
- complications
- abdominal aortic aneurysm
- carotid stenosis
- lower extremity arterial disease
1. Introduction
This state-of-the-art article focuses on screening for the most common arterial diseases (ADs) in the over 50–60-year-old population to prevent dreadful complications using medical, endovascular, or surgical therapy (that is, secondary prevention). ADs start as asymptomatic or paucisymptomatic, and should be searched for when middle-age patients with one or more risk factors for atherosclerosis seek medical consultation, for instance to a cardiologist or a pneumoligist.
2. Abdominal Aortic Aneurysm (AAA)
An artery is defined as an aneurysm if its maximal transverse diameter exceeds the average diameter by at least 50%. Around 80% of the aneurysms of the aorta, exclusively or in part, involve the abdominal portion: compared to thoracic aorta aneurysms, AAAs are easier to diagnose (i.e., by the means of physical examination and duplex scan investigation). Almost 90% of AAAs are localized below the renal arteries (Figure 1).
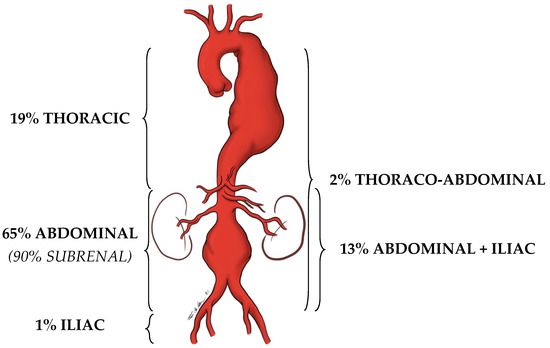
Figure 1. Localization prevalence of aortic aneurysms.
The diameter of the infrarenal aorta in normal adults is in the range of 1.41–2.39 cm in men and 1.19–2.16 cm in women [1]. Therefore, researchers should refer to the diameter of the aorta immediately above the dilation, before eventually declaring that that dilation is effectively an AAA.
AAAs are almost always asymptomatic. Sometimes the patient reports feeling “the heart in the belly” when relaxing in a bed or chair. Endovascular or open treatment is indicated when the maximal transverse diameter reaches or exceeds 5.5 cm. At this dimension, the annual rupture rate (and the consequent fatal hemorrhage) is 5–10% per year, which is higher than the estimated rate of elective operative complications (around 2–5% in dedicated vascular centers). The rupture rate increases proportionally with the growth of the AAA, and a ruptured AAA (rAAA) rarely gives a warning [2].
AAA is more common in males but if present in females presents a higher rupture risk. It often manifests with abdominal or back pain and hypotension: too late to avoid an overall mortality rate that can be up to 80% [3][4]. This is why detecting an asymptomatic intact AAA practically means saving a patient’s life.
3. Extracranial Internal Carotid Stenosis (EICS)
Around 85% of strokes are of ischemic origin. Atrial fibrillation is a major cause of cerebral embolization, causing more transient ischemic attacks (TIAs) than strokes, but 15–20% of all strokes are due to atherosclerotic obstructive lesions of the EICS [5][6]. Therefore, treating EICS due to an atherosclerotic plaque means secondary prevention of cerebral ischemia.
The pathogenetic mechanism is mainly embolic or related to cerebral hypoperfusion in case of severe occlusive disease in both the carotid and vertebral arteries.
4. Lower Extremity Arterial Disease (LEAD)
LEAD is a progressive atherosclerotic occlusion of the arteries of the lower limbs. If not medically treated, according to the natural history of atherosclerosis, LEAD can chronically progress to chronic limb-threatening ischemia (CLTI), a condition characterized by rest pain and gangrene in the foot. Furthermore, according to Virchow’s triad, the endothelial lesions represented by the atherosclerotic plaques can be the sites of overimposed acute arterial thrombosis, so that LEAD can acutely complicate with acute limb ischemia (ALI). Both CLTI and ALI carry a high risk of major adverse cardiovascular events (MACE), that is, acute myocardial infarction and stroke, and major adverse limb events (MALE), up to major amputation [7][8].
Furthermore, LEAD itself is an independent predictor of MACE. Its incidence increases significantly with age, showing a 20% prevalence peak in the over-80 population [9].
Fortunately, if compared to the ADs dealt with before, LEAD is more symptomatic, hence facilitating its diagnosis. However, only one-third of LEAD patients present the typical intermittent claudication (IC), a cramping pain in some muscles of the lower limbs, which arises while climbing stairs or walking and recedes within a few minutes of resting [10].
5. Popliteal Artery Aneurysm (PAA)
The diameter of the popliteal artery in normal adults is in the range of 0.7–1.1 cm in men and 0.5–0.9 cm in women [1]. Therefore, dilation of the popliteal artery is a PAA when its maximal transverse diameter exceeds the standard diameter of the popliteal artery immediately above the dilation by at least 50%.
PAAs should be detected not so much for the risk of rupture, which is rare, but for their risk of embolization and thrombosis. The physiologic flexion of the knee can act as a tremendous stress for the parietal thrombus of the PAA, potentially causing paroxysmal, multiple, and insidious episodes of asymptomatic microembolization to the tibial arteries (Figure 2). As a consequence, the latter can progressively obstruct, drastically reducing the run-off of the popliteal artery and giving rise to a clinical picture ranging from blue toe syndrome, or LEAD with IC up to chronic limb-threatening ischemia. PAA can also thrombose entirely due to the affected tibial out-flow, so it manifests itself with ALI: limb loss can reach 14% in these patients [11][12].
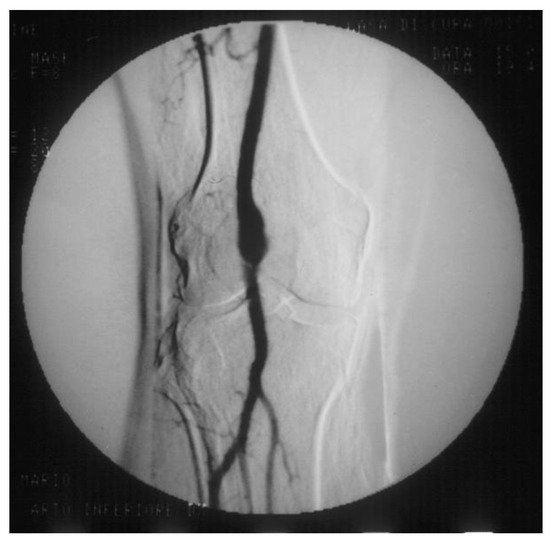
Figure 2. Arteriography showing popliteal artery aneurysm. The physiologic flexion movement of the knee can dislocate part of the mural thrombus, which embolizes and occludes some tibial arteries, giving rise to clinical pictures ranging from an asymptomatic state to intermittent claudication, or chronic limb-threatening ischemia, or acute limb ischemia.
6. Renal Artery Stenosis (RAS)
RAS is associated with renovascular hypertension (RVH) and chronic renal insufficiency (CRI), so it configures the clinical picture of renovascular disease [13]. RAS greater than 50% causes dysregulation of the renin–angiotensin–aldosterone mechanism, which can cause RVH. The differential diagnosis between RVH and essential hypertension is difficult since specific symptoms and signs are lacking. However, RVH should be suspected in some patients since, if not adequately pharmacologically controlled, in the long term, it brings parenchymal alterations, even in the contralateral kidney, with reduced excretory capacity [14].
7. Prevertebral Subclavian Artery Stenosis (PSAS)
A tight atherosclerotic stenosis (or occlusion) at the origin of the subclavian artery before the onset of its first collateral branch, the vertebral artery, can cause a brain stem blood steal during homolateral upper limb efforts, in favor of the latter, through a blood flow inversion in the homolateral vertebral artery itself. This condition is known as subclavian steal syndrome (SSS), which can acutely give rise to dramatic vertebrobasilar symptoms such as vertigo and lipothymia up to syncope (Figure 3).
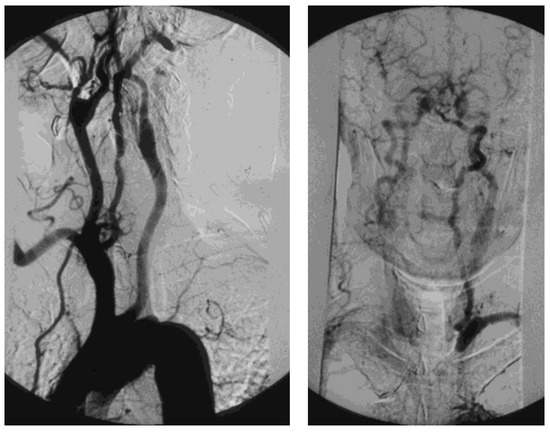
Figure 3. Arteriography showing occlusion of the left prevertebral subclavian artery at its origin (on the left): the late angiogram (on the right) demonstrates the blood flow inversion in the left vertebral artery, revascularizing the left subclavian artery after the occlusion, that is subclavian steal syndrome.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13142356
References
- Johnston, K.W.; Rutherford, R.B.; Tilson, M.D.; Shah, D.M.; Hollier, L.; Stanley, J.C. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J. Vasc. Surg. 1991, 13, 452–458.
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.
- Reimerink, J.J.; van der Laan, M.J.; Koelemay, M.J.; Balm, R.; Legemate, D.A. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br. J. Surg. 2013, 100, 1405–1413.
- Kumar, Y.; Hooda, K.; Li, S.; Goyal, P.; Gupta, N.; Adeb, M. Abdominal aortic aneurysm: Pictorial review of common appearances and complications. Ann. Transl. Med. 2017, 5, 256.
- Arboix, A.; Oliveres, M.; Massons, J.; Pujades, R.; Garcia-Eroles, L. Early differentiation of cardioembolic from atherothrombotic cerebral infarction: A multivariate analysis. Eur. J. Neurol. 1999, 6, 677–683.
- Chaturvedi, S.; Bruno, A.; Feasby, T.; Holloway, R.; Benavente, O.; Cohen, S.N.; Cote, R.; Hess, D.; Saver, J.; Spence, J.D.; et al. Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Carotid endarterectomy–An evidence-based review: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005, 65, 794–801.
- Heart Protection Study Collaborative Group. Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: A randomised controlled trial. Lancet 2011, 378, 2013–2020.
- Gabel, J.; Jabo, B.; Patel, S.; Kiang, S.; Bianchi, C.; Chiriano, J.; Teruya, T.; Abou-Zamzam, A.M., Jr. Vascular Quality Initiative. Analysis of Patients Undergoing Major Lower Extremity Amputation in the Vascular Quality Initiative. Ann. Vasc. Surg. 2018, 46, 75–82.
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340.
- Grøndal, N.; Søgaard, R.; Lindholt, J.S. Baseline prevalence of abdominal aortic aneurysm, peripheral arterial disease and hypertension in men aged 65-74 years from a population screening study (VIVA trial). Br. J. Surg. 2015, 102, 902–906.
- Martelli, E.; Ippoliti, A.; Ventoruzzo, G.; De Vivo, G.; Ascoli Marchetti, A.; Pistolese, G.R. Popliteal artery aneurysms. Factors associated with thromboembolism and graft failure. Int. Angiol. 2004, 23, 54–65.
- Kropman, R.H.; Schrijver, A.M.; Kelder, J.C.; Moll, F.L.; de Vries, J.P. Clinical outcome of acute leg ischaemia due to thrombosed popliteal artery aneurysm: Systematic review of 895 cases. Eur. J. Vasc. Endovasc. Surg. 2010, 39, 452–457.
- Schwartz, C.J.; White, T.A. Stenosis of renal artery: An unselected necropsy study. Br. Med. J. 1964, 2, 1415–1421.
- Safian, R.D.; Textor, S.C. Renal-artery stenosis. N. Engl. J. Med. 2001, 344, 431–442.
This entry is offline, you can click here to edit this entry!
