Anthocyanins, the colored water-soluble pigments, have increasingly drawn the attention of researchers for their novel applications. The sources of anthocyanin are highly diverse, and it can be easily extracted. The unique biodiversity of the Himalayan Mountain range is an excellent source of anthocyanin, but it is not completely explored. Numerous attempts have been made to study the phytochemical aspects of different Himalayan plants. In this entry, some Himalayan sources of anthocyanins have been described to create a path for further research and sustainable utilization.
- anthocyanin
- himalayan plant
- natural colorant
- food
- nutraceuticals
- botany
- plantsciences
1. Introduction
2. Chemistry of Anthocyanins
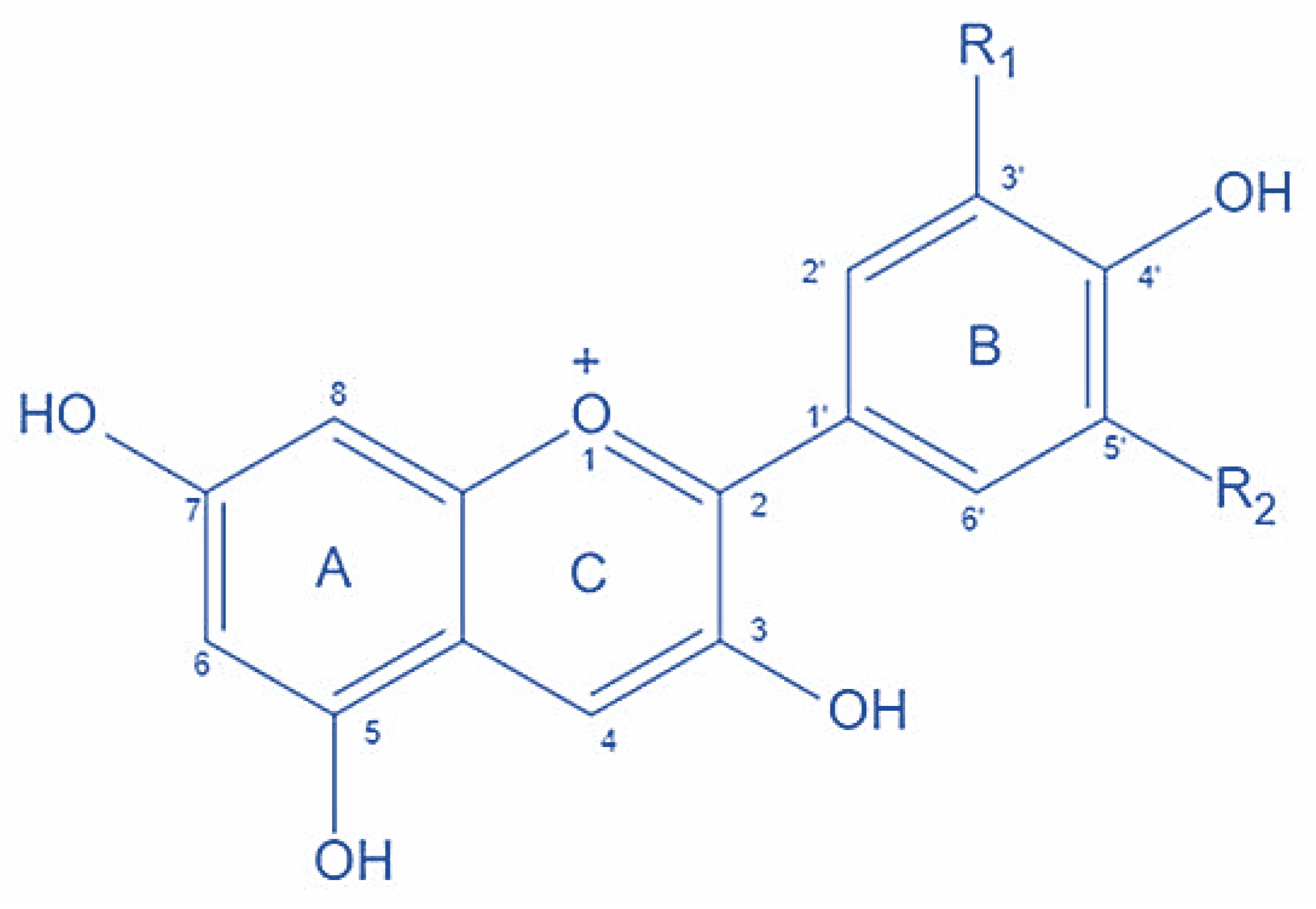
3. Extraction of Anthocyanins
4. Himalayan Sources of Anthocyanins
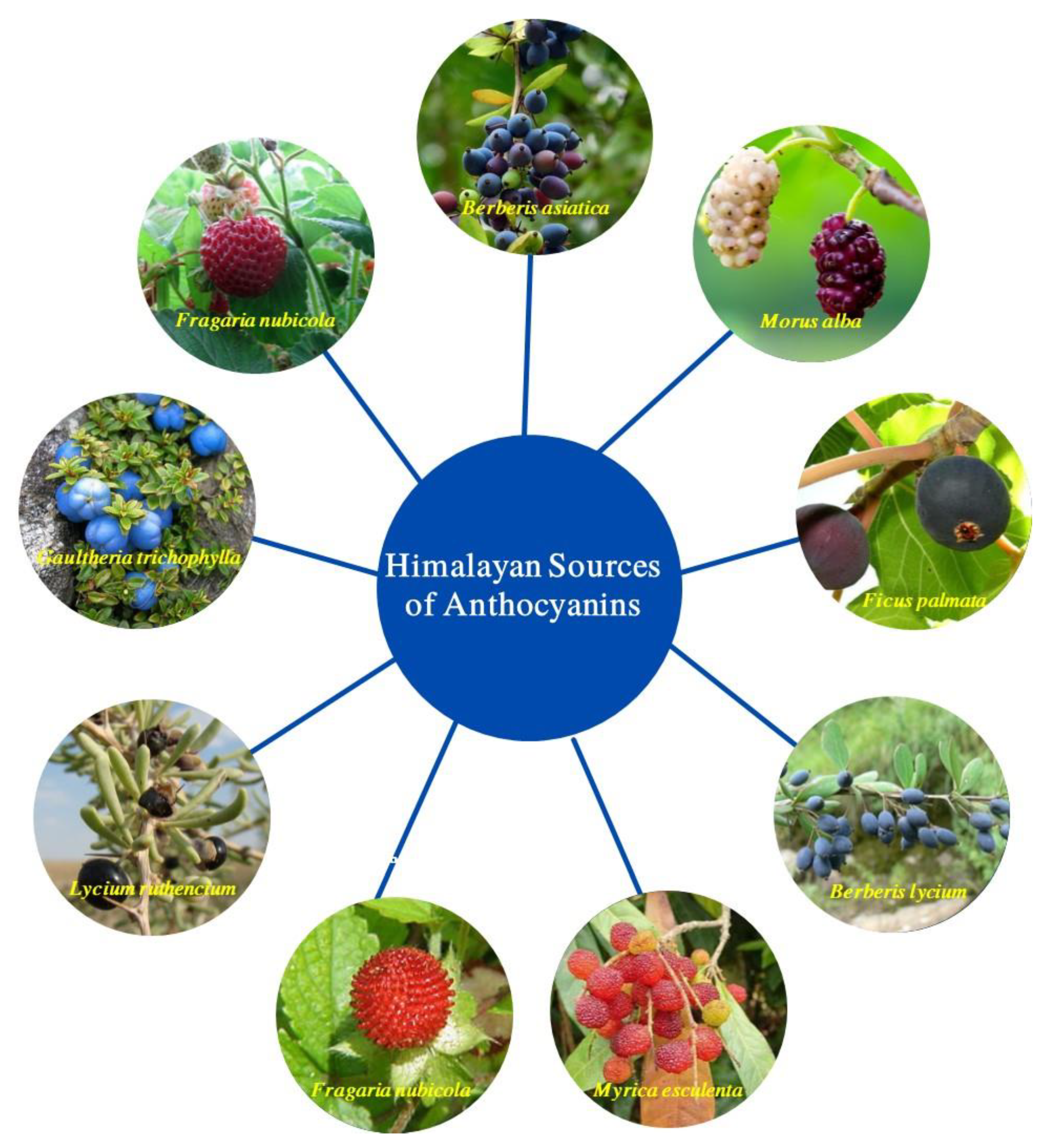
4.1. Berberis asiatica
4.2. Morus alba
4.3. Ficus palmata
4.4. Berberis lycium
4.5. Myrica esculenta
4.6. Duchesnea indica
4.7. Lycium ruthencium
4.8. Gaultheria trichophylla
4.9. Species of Genus Begonia
4.10. Fragaria nubicola
5. Conclusions
Anthocyanins are natural colorants that are gaining popularity due to their diverse color palette as well as safe and favorable health effects. Anthocyanins have a high potential for application in food, pharmaceutical, cosmetic, and related industries. Anthocyanin can be isolated and purified from an endless number of natural resources, but the Himalayan sources of anthocyanin are relatively undiscovered. Most anthocyanin research is currently focused on identifying different sources of anthocyanin, as well as purification and extraction. Natural food colorants are recently being preferred by consumers since they have fewer adverse effects than synthetic/artificial substances. Himalayan plants are rich sources of anthocyanins.
This entry is adapted from the peer-reviewed paper 10.3390/foods12112203
References
- Kumar, V.; Chopra, A.K. Impact of Climate Change on Biodiversity of India with Special Reference to Himalayan Region-An Overview. J. Appl. Nat. Sci. 2009, 1, 117–122.
- Delgoda, R.; Murray, J.E. Evolutionary Perspectives on the Role of Plant Secondary Metabolites. In Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 93–100.
- Oz, A.T.; Kafkas, E. Phytochemicals in Fruits and Vegetables. In Superfood and Functional Food—An Overview of Their Processing and Utilization; InTech: Sydney, Australia, 2017; pp. 175–184.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47.
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Micro and Nanoencapsulation of Natural Colors: A Holistic View. Appl. Biochem. Biotechnol. 2021, 193, 3787–3811.
- Ren, S.; Jiménez-Flores, R.; Giusti, M.M. The Interactions between Anthocyanin and Whey Protein: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5992–6011.
- Cruz, L.; Fernandes, I.; Guimarães, M.; de Freitas, V.; Mateus, N. Enzymatic Synthesis, Structural Characterization and Antioxidant Capacity Assessment of a New Lipophilic Malvidin-3-Glucoside–Oleic Acid Conjugate. Food Funct. J. 2016, 7, 2754–2762.
- Luo, C.-L.; Zhou, Q.; Yang, Z.-W.; Wang, R.-D.; Zhang, J.-L. Evaluation of Structure and Bioprotective Activity of Key High Molecular Weight Acylated Anthocyanin Compounds Isolated from the Purple Sweet Potato (Ipomoea Batatas L. Cultivar Eshu No. 8). Food Chem. 2018, 241, 23–31.
- Yang, W.; Kortesniemi, M.; Ma, X.; Zheng, J.; Yang, B. Enzymatic Acylation of Blackcurrant (Ribes nigrum) Anthocyanins and Evaluation of Lipophilic Properties and Antioxidant Capacity of Derivatives. Food Chem. 2019, 281, 189–196.
- Farooq, S.; Shah, M.A.; Siddiqui, M.W.; Dar, B.N.; Mir, S.A.; Ali, A. Recent Trends in Extraction Techniques of Anthocyanins from Plant Materials. J. Food Meas. Charact. 2020, 14, 3508–3519.
- Weber, F.; Boch, K.; Schieber, A. Influence of Copigmentation on the Stability of Spray Dried Anthocyanins from Blackberry. LWT-Food Sci. Technol. 2017, 75, 72–77.
- Ongkowijoyo, P.; Luna-Vital, D.A.; Gonzalez de Mejia, E. Extraction Techniques and Analysis of Anthocyanins from Food Sources by Mass Spectrometry: An Update. Food Chem. 2018, 250, 113–126.
- del Pilar Garcia-Mendoza, M.; Espinosa-Pardo, F.A.; Baseggio, A.M.; Barbero, G.F.; Maróstica Junior, M.R.; Rostagno, M.A.; Martínez, J. Extraction of Phenolic Compounds and Anthocyanins from Juçara (Euterpe Edulis Mart.) Residues Using Pressurized Liquids and Supercritical Fluids. J. Supercrit. Fluids 2017, 119, 9–16.
- Ahrendt, L.W.A. Berberis and Mahonia. Bot. J. Linn. Soc. 1961, 57, 1–410.
- Srivastava, S.K.; Singh Rawat, A.K.; Mehrotra, S. Pharmacognostic Evaluation of the Root of Berberis asiatica. Pharm. Biol. 2004, 42, 467–473.
- Leopoldini, M.; Russo, N.; Toscano, M. The Molecular Basis of Working Mechanism of Natural Polyphenolic Antioxidants. Food Chem. 2011, 125, 288–306.
- Potdar, D.; Hirwani, R.R.; Dhulap, S. Phyto-Chemical and Pharmacological Applications of Berberis aristata. Fitoterapia 2012, 83, 817–830.
- Belwal, T.; Pandey, A.; Bhatt, I.D.; Rawal, R.S.; Luo, Z. Trends of Polyphenolics and Anthocyanins Accumulation along Ripening Stages of Wild Edible Fruits of Indian Himalayan Region. Sci. Rep. 2019, 9, 5894.
- Cicero, A.F.G.; Baggioni, A. Berberine and Its Role in Chronic Disease. In Anti-Inflammatory Nutraceuticals and Chronic Diseases; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; pp. 27–45.
- Bhatt, I.D.; Rawat, S.; Badhani, A.; Rawal, R.S. Nutraceutical Potential of Selected Wild Edible Fruits of the Indian Himalayan Region. Food Chem. 2017, 215, 84–91.
- Srivastava, S.; Kapoor, R.; Thathola, A.; Srivastava, R.P. Nutritional Quality of Leaves of Some Genotypes of Mulberry (Morus alba). Int. J. Food Sci. Nutr. 2006, 57, 305–313.
- Awasthi, A.K.; Nagaraja, G.; Naik, G.; Kanginakudru, S.; Thangavelu, K.; Nagaraju, J. Genetic Diversity and Relationships in Mulberry (Genus Morus) as Revealed by RAPD and ISSR Marker Assays. BMC Genet. 2004, 5, 1.
- Yadav, S.; Nair, N.; Biharee, A.; Prathap, V.M.; Majeed, J. Updated Ethnobotanical Notes, Phytochemistry and Phytopharmacology of Plants Belonging to the Genus Morus (Family: Moraceae). Phytomed. Plus 2022, 2, 100120.
- Chan, E.W.-C.; Lye, P.-Y.; Wong, S.-K. Phytochemistry, Pharmacology, and Clinical Trials of Morus alba. Chin. J. Nat. Med. 2016, 14, 17–30.
- Ercisli, S.; Orhan, E. Chemical Composition of White (Morus alba), Red (Morus rubra) and Black (Morus nigra) Mulberry Fruits. Food Chem. 2007, 103, 1380–1384.
- Kumar, R.; And, R.; Chauhan, S. Mulberry: Life Enhancer. J. Med. Plants Res. 2008, 2, 271–278.
- Gryn-Rynko, A.; Bazylak, G.; Olszewska-Slonina, D. New Potential Phytotherapeutics Obtained from White Mulberry (Morus alba L.) Leaves. Biomed. Pharmacother. 2016, 84, 628–636.
- Kimura, T.; Nakagawa, K.; Kubota, H.; Kojima, Y.; Goto, Y.; Yamagishi, K.; Oita, S.; Oikawa, S.; Miyazawa, T. Food-Grade Mulberry Powder Enriched with 1-Deoxynojirimycin Suppresses the Elevation of Postprandial Blood Glucose in Humans. J. Agric. Food Chem. 2007, 55, 5869–5874.
- Kumari, K.; Sharma, S.; Kaushik, R. Wild Himalayan Fig: A Nutraceutical under exploited fruit of Western Himalayan region—A Review. Int. J. Adv. Res. 2017, 5, 833–839.
- Joshi, Y.; Joshi, A.K.; Prasad, N.; Juyal, D. A Review on Ficus palmata (Wild Himalayan Fig). J. Phytopharm. 2014, 3, 374–377.
- Kothiyal, S.C.; Saklani, S. Isolation, and Identification of Ficus palmata leaves and their antimicrobial activities. J. Sci. Res. 2017, 9, 193–200.
- Saklani, S.; Kothiyal, S. Phytochemical Screening of Garhwal Himalaya Wild Edible Fruit Ficus palmata. Int. J. Pharm. Tech Res. 2012, 4, 1185–1191.
- Shabbir, A. Berberis lycium Royle: A Review of Its Traditional Uses, Phytochemistry and Pharmacology. Afr. J. Pharm. Pharmacol. 2012, 6, 2346–2353.
- Anjum, N.; Ridwan, Q.; Akhter, F.; Hanief, M. Phytochemistry and Therapeutic Potential of Berberis lycium Royle; an Endangered Species of Himalayan Region. Acta Ecol. Sin. 2022, 43, 577–584.
- Kaur, C.; Kapoor, H.C. Antioxidants in Fruits and Vegetables—The Millennium’s Health. Int. J. Food Sci. Technol. 2008, 36, 703–725.
- Gupta, M.; Singh, A.; Joshi, H. Berberis lycium Multipotential Medicinal Application: An Overview. Int. J. Chem. Stud. 2015, 10, 10–13.
- Bhardwaj, D.; Kaushik, N. Phytochemical and Pharmacological Studies in Genus Berberis. Phytochem. Rev. 2012, 11, 523–542.
- Gulfraz, M.; Mehmood, S.; Ahmad, A.; Fatima, N.; Praveen, Z.; Williamson, E.M. Comparison of the Antidiabetic Activity of Berberis lycium Root Extract and Berberine in Alloxan-Induced Diabetic Rats. Phytother. Res. 2008, 22, 1208–1212.
- Nazir, N.; Rahman, A.; Uddin, F.; Khan Khalil, A.A.; Zahoor, M.; Nisar, M.; Ullah, S.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.E. Quantitative Ethnomedicinal Status and Phytochemical Analysis of Berberis lycium Royle. Agronomy 2021, 11, 130.
- Sendri, N.; Bhandari, P. Polyphenolic Composition and Antioxidant Potential of Underutilized Himalayan Wild Edible Berries by High-performance Liquid Chromatography Coupled with Electrospray Ionization Quadrupole Time-of-flight Mass Spectrometry. J. Sep. Sci. 2021, 44, 4237–4254.
- Pradhan, P.C.; Saha, S. Anthocyanin Profiling of Berberis lycium Royle Berry and Its Bioactivity Evaluation for Its Nutraceutical Potential. J. Food Sci. Technol. 2016, 53, 1205–1213.
- Yanthan, M.; Biate, D.; Misra, A.K. Taxonomic Resolution of Actinorhizal Myrica Species from Meghalaya (India) through Nuclear RDNA Sequence Analyses. Funct. Plant Biol. 2011, 38, 738.
- Gusain, Y.S.; Khanduri, V.P. Myrica esculenta Wild Edible Fruit of Indian Himalaya: Need a Sustainable Approach for Indigenous Utilization. Ecol. Environ. Conserv. J. 2016, 22, S267–S270.
- Shri, R.; Sood, P. A Review on Ethnomedicinal, Phytochemical and Pharmacological Aspects of Myrica esculenta. Indian J. Pharm. Sci. 2018, 80, 2–13.
- Rawat, S.; Jugran, A.; Giri, L.; Bhatt, I.D.; Rawal, R.S. Assessment of Antioxidant Properties in Fruits of Myrica esculenta: A Popular Wild Edible Species in Indian Himalayan Region. Evid.-Based Complement. Altern. Med. 2011, 2011, 51278.
- Kabra, A.; Martins, N.; Sharma, R.; Kabra, R.; Baghel, U.S. Myrica esculenta Buch.-Ham. Ex D. Don: A Natural Source for Health Promotion and Disease Prevention. Plants 2019, 8, 149.
- Nhiem, N.X.; Van Kiem, P.; Van Minh, C.; Tai, B.H.; Cuong, N.X.; Thu, V.K.; Anh, H.L.T.; Jo, S.-H.; Jang, H.-D.; Kwon, Y.-I.; et al. A New Monoterpenoid Glycoside from Myrica esculenta and the Inhibition of Angiotensin I-Converting Enzyme. Chem. Pharm. Bull. 2010, 58, 1408–1410.
- Bahuguna, D.P.; London, H.K.; Kharwal, H.; Joshi, D. Myrica Nagi: A Review on Active Constituents, Biological and Therapeutic Effects. Int. J. Pharm. Pharm. Sci. 2012, 4, 38–42.
- Kumar, T.; Pande, K.K.; Sharma, H.; Koranga, M.; Pande, L. HRLC-ESI-MS Based Separation, and Identification of Anthocyanins Extracted from Popular Wild Edible Fruit of Himalaya: Myrica esculenta (Himalayan Bayberry). J. Adv. Sci. Res. 2020, 11, 269–275.
- Kakar, M.; Kakar, I.; Akram, M. Antimicrobial and Phytochemical Exploration of Duchesnea indica Plant. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 74–85.
- Faghir, M.B.; Pourebrahim, S.; Shahi Shavvon, R. New Insight into the Molecular and Micromorphological Characteristics of Potentilla indica and Potentilla reptans (Rosaceae). Iran. J. Bot. 2022, 28, 77–95.
- Kar, T.; Nayak, A.K.N.A.K.; Dash, B.; Mandal, K.K.M.K.K. Duchesnea indica (Rosaceae): An Addition to the Flora of Odisha, India. Biosci. Discov. 2014, 5, 202–203.
- Ahmad, I.; Ibrar, M.; Barkatullah; Ali, N. Ethnobotanical Study of Tehsil Kabal, Swat District, KPK, Pakistan. J. Bot. 2011, 2011, 368572.
- Peng, B.; Chang, Q.; Wang, L.; Hu, Q.; Wang, Y.; Tang, J.; Liu, X. Suppression of Human Ovarian SKOV-3 Cancer Cell Growth by Duchesnea Phenolic Fraction Is Associated with Cell Cycle Arrest and Apoptosis. Gynecol. Oncol. 2008, 108, 173–181.
- Zhu, M.; Dong, X.; Guo, M. Phenolic Profiling of Duchesnea indica Combining Macroporous Resin Chromatography (MRC) with HPLC-ESI-MS/MS and ESI-IT-MS. Molecules 2015, 20, 22463–22475.
- Qin, C.; Li, Y.; Zhang, R.; Niu, W.; Ding, Y. Separation and Elucidation of Anthocyanins in the Fruit of Mockstrawberry (Duchesnea indica Focke). Nat. Prod. Res. 2009, 23, 1589–1598.
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Duan, J.A. Lycium ruthenicum studies: Molecular biology, phytochemistry and pharmacology. Food Chem. 2018, 240, 759–766.
- Sharma, R.; Raghuvanshi, R.; Kumar, R.; Thakur, M.S.; Kumar, S.; Patel, M.K.; Chaurasia, O.P.; Saxena, S. Current Findings and Future Prospective of High-Value Trans Himalayan Medicinal Plant Lycium ruthenicum Murr: A Systematic Review. Clin. Phytosci. 2022, 8, 3.
- Chaurasia, O.; Ballabh, B. Herbal Formulations from Cold Desert Plants Used for Gynecological Disorders. Ethnobot. Res. Appl. 2011, 9.
- Proksch, P. Chinese Marine Materia Medica. By Huashi Guan and Shuguang Wang. Shanghai Scientific and Technical Publishers, China Ocean Press, and Chemical Industry Press: Shanghai, Beijing, China, 2009. Mar. Drugs 2014, 12, 193–195.
- Ballabh, B.; Chaurasia, O.P.; Ahmed, Z.; Singh, S.B. Traditional Medicinal Plants of Cold Desert Ladakh—Used against Kidney and Urinary Disorders. J. Ethnopharmacol. 2008, 118, 331–339.
- Chopra, R.N. Glossary of Indian Medicinal Plants; Council of Scientific & Industrial Research: New Delhi, India, 1956.
- Gairola, S.; Sharma, J.; Bedi, Y.S. A Cross-Cultural Analysis of Jammu, Kashmir and Ladakh (India) Medicinal Plant Use. J. Ethnopharmacol. 2014, 155, 925–986.
- Yun, D.; Yan, Y.; Liu, J. Isolation, structure and biological activity of polysaccharides from the fruits of Lycium ruthenicum murr: A review. Carbohydr. Polym. 2022, 291, 119618.
- Liu, W.-R.; Qiao, W.-L.; Liu, Z.-Z.; Wang, X.-H.; Jiang, R.; Li, S.-Y.; Shi, R.-B.; She, G.-M. Gaultheria: Phytochemical and Pharmacological Characteristics. Molecules 2013, 18, 12071–12108.
- Alam, F.; Saqib, Q.N.; Ashraf, M. Gaultheria trichophylla (Royle): A Source of Minerals and Biologically Active Molecules, Its Antioxidant and Anti-Lipoxygenase Activities. BMC Complement. Altern. Med. 2017, 17, 3.
- Alam, F.; Najum us Saqib, Q. Pharmacognostic Standardization and Preliminary Phytochemical Studies of Gaultheria trichophylla. Pharm. Biol. 2015, 53, 1711–1718.
- Zhang, D.; Liu, R.; Sun, L.; Huang, C.; Wang, C.; Zhang, D.-M.; Zhang, T.-T.; Du, G.-H. Anti-Inflammatory Activity of Methyl Salicylate Glycosides Isolated from Gaultheria yunnanensis (Franch.) Rehder. Molecules 2011, 16, 3875–3884.
- Bahukh, A.; Aseesh, P.; Sekar, K.C.; Bhatt, I.D. Polyphenolics, Nutrients and Antioxidant Activity of Gaultheria trichophylla Royle: A High Value Wild Edible Plant of Trans Himalaya. Hortic. Int. J. 2017, 1, 39–43.
- Moonlight, P.W.; Ardi, W.H.; Padilla, L.A.; Chung, K.-F.; Fuller, D.; Girmansyah, D.; Hollands, R.; Jara-Muñoz, A.; Kiew, R.; Leong, W.-C.; et al. Dividing and Conquering the Fastest–Growing Genus: Towards a Natural Sectional Classification of the Mega–Diverse Genus Begonia (Begoniaceae). Taxon 2018, 67, 267–323.
- Iwashina, T.; Saito, Y.; Kokubugata, G.; Peng, C.-I. Flavonoids in the Leaves of Hillebrandia and Begonia Species (Begoniaceae). Biochem. Syst. Ecol. 2020, 90, 104040.
- Taram, M.; Borah, D.; Hughes, M. Two New Records of Begonia for the Flora of India from Arunachal Pradesh. Phytotaxa 2023, 584, 2.
- Bhattarai, B.; Rana, M. Diversified Morphological and Phytochemical Screening of Wild Begonia of Sikkim Himalaya. Ecol. Environ. Conserv. 2020, 26, S129–S138.
- Bhutia, P.O.; Kewlani, P.; Pandey, A.; Rawat, S.; Bhatt, I.D. Physico-Chemical Properties and Nutritional Composition of Fruits of the Wild Himalayan Strawberry (Fragaria nubicola Lindle.) in Different Ripening Stages. J. Berry Res. 2021, 11, 481–496.
- Roshan, R.; Ahmed, S.; ul Hassan, M.M. Fragaria nubicola (Rosaceae): A Review of Medicinal Uses, Phytochemistry and Pharmacology. J. Pharmacogn. Phytochem. 2019, 8, 3390–3393.
- Staudt, G. Himalayan Species of Fragaria (Rosaceae). Bot. JahrbÜCher Syst. Pflanzengesch. Pflanzengeogr. 2006, 126, 483–508.
- Chakraborty, T.; Saha, S.; Bisht, N. First Report on the Ethnopharmacological Uses of Medicinal Plants by Monpa Tribe from the Zemithang Region of Arunachal Pradesh, Eastern Himalayas, India. Plants 2017, 6, 13.
- Antonio, R.L.; Kozasa, E.H.; Galduróz, J.C.F.; Dawa; Dorjee, Y.; Kalsang, T.; Norbu, T.; Tenzin, T.; Rodrigues, E. Formulas Used by Tibetan Doctors at Men-Tsee-Khang in India for the Treatment of Neuropsychiatric Disorders and Their Correlation with Pharmacological Data. Phytother. Res. 2013, 27, 552–563.
- Thakur, P. Sarika, Ethno-Medicinal Uses of Some Plants of Potter’s Hill in Shimla (Himachal Pradesh, India). Proc. Biol. Forum 2016, 8, 417–422.
- Rakhunde, P.B.; Ali, S.A. Antioxidant and Cytoprotective Effect of Fragaria nubicola on Ischemia Reperfusion Induced Brain Injury. Ann. Exp. Biol. 2014, 2, 33–38.
- Bahukhandi, A.; Barola, A.; Sekar, K.C. Antioxidant Activity and Polyphenolics of Fragaria nubicola: A Wild Edible Fruit Species of Himalaya. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 761–767.
