Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Dentistry, Oral Surgery & Medicine
Peri-implantitis is a multi-factorial disease with an inflammatory background that occurs in both soft and hard tissues surrounding implants. A wide array of cells stands behind peri-implantitis, as well as cytokines and their genetic variations that take part in the process. Recently, growing interest in this topic has led to the introduction of specific new diagnostic tools to enable a better understanding of patients’ responses to treatment and, in turn, to even enable prediction of the risk of developing peri-implant disease.
- inflammation
- peri-implantitis
- cellular response
- molecular factors
- genetic polymorphism
- cytokines
1. Introduction
The wide spread of prosthetic restorations based on dental implants enables optimal oral rehabilitation of totally and partially edentulous patients, expanding the available treatment possibilities. Currently, the most common implant materials are pure titanium, Ti-6Al-4V alloy and zirconia. Additional modifications of the implant surface, for example, acid etching and sandblasting or coating, enhance the osseointegration process, extend the bone–implant contact area and reduce the risk of implant failure [1]. The prevalence of dental implants in the global population is estimated to reach up to 23% by the year 2026 [2]. The growing number of patients translates to a higher number of potential peri-implant complications. One of these is peri-implantitis. It is defined as a progressive, irreversible disease affecting both hard (alveolar bone) and soft tissues (supracrestal tissues and mucosa) surrounding dental implants. The amount of keratinized mucosa, the supracrestal tissue height and the peri-implant bone thickness can all affect peri-implantitis occurrence [3]. Additionally, in peri-implantitis, there can be bone loss, hindered implant osseointegration and pathological pocket formation [4]. It is commonly associated with bacterial challenge inducing the inflammatory process in surrounding soft tissues and loss of bone support. Like periodontitis, the main bacteria responsible for the development of peri-implantits belong to Socransky’s red complex. These are Porphyromonas gingivalis, Tannarella forsythia and Treponema denticola [5], however the range of suspected bacteria involved in the development of peri-implant pockets is broader. Often found microbes in the peri-implant pockets are often not specific, but commonly present, like Campylobacter, Gemella, Bacteroides, Actinomyces, Peptostreptococcus, Streptococcus, Candida, Treponema, E. corrodes and P. nigrescens [6]. While the microbial flora in peri-implantitis is relatively well known, much less information is available regarding the cellular and molecular responses. In medicine, cytokines are widely used as diagnostic and prognostic tools [7]. They are used to monitor the status of patients undergoing treatment for asthma, cancer, AIDS, heart disease, degenerative diseases and rheumatoid arthritis, to name only a few of them [7,8,9,10,11]. In dentistry, the use of cytokines is uncommon, only recently becoming more widespread [12]. The fields of dentistry that utilize them are periodontics and implantology. An increase in implant surgeries and implant-supported restorations has led to a higher incidence of peri-implant disease occurrence in the population. The most-investigated biomarkers in periodontal and peri-implant tissues are IL-1β, VEGF, MMP-8, TIMP-2 and OPG, as they alter soft and hard tissue cellular metabolism and seem to have different mean local concentrations during bacterial infections [13,14,15,16,17]. Peri-implant cervical fluid (PICF) is used as a site-specific and easy-to-obtain fluid. PICF is similar to gingival cervical fluid exerted from the gingival sulcus and contains cells, bacteria, cytokines and active mediators [18]. To collect PICF, sterile paper strips are placed within the sulcus or peri-implant crevice and held for 30 s to properly soak up the fluid [19]. It is important that the paper strips do not become contaminated with blood or pus. The strips are then placed in tubes containing buffered saline and phenylmethylsulfonyl fluoride and centrifuged [19,20]. PICF is further used to conduct ELISA tests for proteins and PCR for DNA and RNA or to perform oral-based point-of-care (PoC) tests [21]. Currently, the ELISA test is the most widely used.
2. Types of Peri-Implant Disease and Criteria for Implant Health and Peri-Implantitis
The peri-implant disease has been divided into three separate categories by the consensus report of the 4th workgroup of the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions (Table 2). These are, respectively: peri-implant health, peri-implant mucositis and peri-implantitis [1]. This division allows for clearer distinction and easier treatment planning, as peri-implantitis is not always coexistent with visible inflammation and can be easily mistaken for peri-implant mucositis. Implant success is recognized differently by different authors. Various definitions have been formulated ever since the first osseointegration cases were published by Branemark et al. Buser et al. [2] defined implant success as lack of mobility, no noticeable radiolucency around the implant, <2 mm of crestal bone loss in the first year of functioning and no inflammatory symptoms. Furthermore, the authors highlighted the importance of the feasibility of restorations. Implant success definition was then further redefined in 2008, at the International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference, as no pain or tenderness upon function, the absence of mobility, <2 mm radiographic bone loss from the initial surgery and no presence of exudate [3]. To date, the most recent definition of peri-implantitis includes the presence of bleeding/suppuration on probing, increasing probing depth between examinations, and crestal bone loss not caused by the initial remodelling [4][5]. The 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions formulated the following definition of peri-implantitis: “peri-implantitis is a plaque-associated pathological condition occurring in tissues around dental implants, characterized by inflammation in the peri-implant mucosa and subsequent progressive loss of supporting bone” [6][7]. Additionally, there are criteria for diagnosing peri-implantitis without previous radiographic and clinical implant history, which consist of a lack of bleeding/suppuration on probing, PD ≥ 6mm and bone levels ≥ 3 mm apical of the most coronal portion of the intraosseous part of the implant [1][4][5][7][8][9][10].
Table 2. Healthy peri-implant vs. peri-implantitis according to 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions.
| Peri-Implant Health | Peri-Implantitis | Peri-Implantitis in the Absence of Previous Examinations |
|---|---|---|
| No clinical sign of inflammation | No sign or visible inflammation | No sign or visible inflammation |
| No bleeding/suppuration on gentle probing | Bleeding/suppuration on gentle probing | Bleeding/suppuration on gentle probing |
| Stable probing depth between examinations | Increased probing depth compared to previous examinations | Probing depth ≥ 6 mm |
| No crestal bone changes apart from initial bone remodelling | Crestal bone loss other than initial bone remodelling | Bone levels ≥ 3 mm apical of the most coronal portion of the intraosseous part of the implant |
3. Risk Factors Associated with Peri-Implantitis
The risk factor of peri-implantitis is dependent not only on individual host susceptibility but also on other factors with various degrees of concurrence. To what extent the risk factors will influence the appearance of peri-implantitis also depends on the frequency, intensity, and individual vulnerability to the factor (i.e., a thicker peri-implant phenotype performs better) [11] and the cooperation of factors acting together. The risk factors include smoking, alcohol drinking, metabolic diseases (e.g., diabetes), previously recognized periodontitis [12][13][14][15][16][17], a poor level of oral hygiene, insufficiently frequent controls, an external implant–abutment connection type and inadequate screw-in torque [12], viral infections (HPV, HHV-4, HHV-6, HHV-7 and COVID-19) [17][18][19], genetic burdens (i.e., Papillon–Lefevre syndrome), titanium particles present after implant placement, and tissue response to prosthetic restoration [20][21]. Smoking is positively correlated with peri-implant disease as it can contribute to hindering the bone blood supply and lower the cellular immunological response and MMP-8 [11][13][15][21][22]. Alcohol drinking can also increase the risk of peri-implantitis, mainly in conjunction with smoking, highlighting the additive influence of both [11][13]. There is also a statistically significant risk of peri-implantitis in obese patients because of higher C-reactive protein and MMP-8 levels in the serum and PICF [21][22]. Patient compliance also plays an important role in the quick detection and effective management of implant tissues [13]. Patients with genetic conditions that can influence periodontal health, such as Papillon–Lefevre syndrome, are inherently more prone to peri-implantitis. In this group, despite the much higher risk, regular clinical controls either lowered or prevented peri-implantitis and its progress [23]. Regarding endocrine malfunctions, diabetes mellitus leads the way. Its growing significance comes from an ever-growing population of patients and new dependencies found in metabolic pathways and genetic connections. In peri-implantitis accompanying diabetes, the main reasons seem to be increased HbA1c and advanced glycemic end product (AGE) levels, which interfere with immune response, bone remodelling (promoting osteoclastogenesis), vascularization, cell apoptosis and inflammation [11][14][21][24]. Risk factors are listed in Figure 2.

Figure 2. Risk factors associated with peri-implantitis.
4. Molecular Factors Contributing to Peri-Implantitis Development
Cytokines are proteins secreted by leukocytes and serve a mainly communicatory role. They influence either pro- or anti-inflammatory responses. In peri-implantitis, the balance between pro-and anti-inflammatory cytokines is disrupted in favour of pro-inflammatory. The most well-known are pro-inflammatory IL-6, IL-1 and TNFα [25]. The most common pro- and anti-inflammatory cytokines are listed in Table 3 with their effects in peri-implantits in Figure 3 and Figure 4. The basic functions of the most common cytokines in peri-implantitis are listed in Table 4.
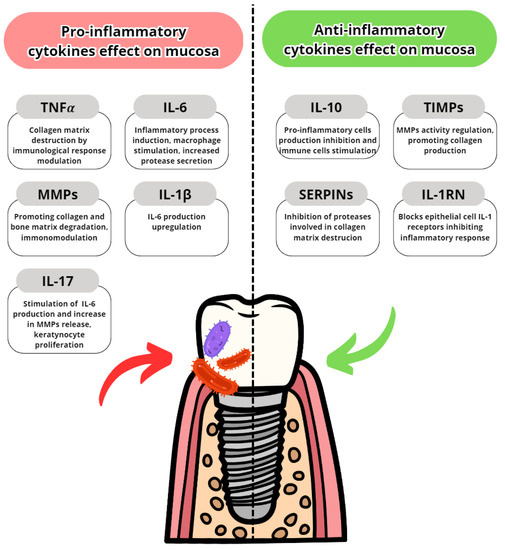
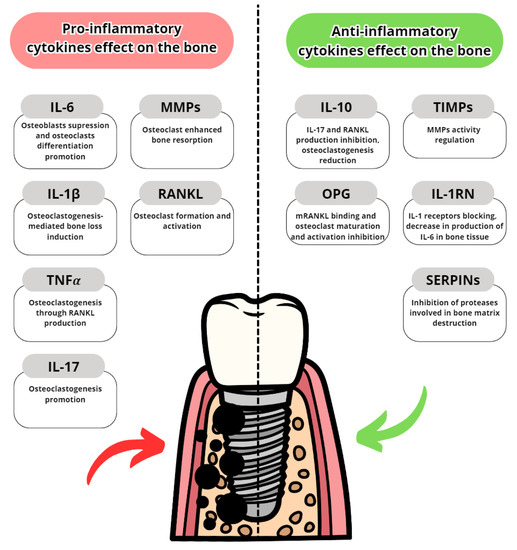
Table 3. Pro- and anti-inflammatory cytokines.
| Pro-Inflammatory Cytokines | Anti-Inflammatory Cytokines |
|---|---|
| Interleukin-6 | Interleukin-10 |
| Interleukin-1 | Tissue Metalloproteinase Inhibitors (TIMPs) |
| Tumor Necrosis Factor α | Osteoprotegrin |
| Interleukin-8 | Interleukin-1RN |
| Interleukin-17 | Serase Protease Inhibitors (SERPINs) |
| Metalloproteinase-8 (MMP-8) and other MMPs |
| Cytokine | Function |
|---|---|
| Interleukin-6 | Stimulating acute phase protein synthesis, neutrophils production, fever mediation, B-cell growth stimulation |
| Interleukin-1α | Part of the epithelial barrier, epithelium integrity preservation |
| Interleukin-1β | Modulating inflammatory response, pyrogen, pain hypersensitivity, cell proliferation |
| Tumor Necrosis Factor α | Immune cells modulation, cell signalling, inflammation regulation, response to bacterial lipopolysaccharide |
| Interleukin-8 | Neutrophil chemotaxis, phagocytosis stimulation |
| Interleukin-17 | Recruitment of immune cells (mainly neutrophils and monocytes) via chemokines, promotes inflammatory responses of IL-1β and TNF-α |
| Interleukin-10 | Anti-inflammatory agent, blocks NFkB activity resulting in a decrease in osteoclast formation, TNF-α regulation |
| MMP-8 | Catalyzes the degradation of collagen type III and I |
| MMP-2 | Collagen type IV degradation, cell-cell clustering |
| MMP-9 | Collagen type IV and V degradation, cooperation with MMP-2 in ECM remodelling |
| MMP-7 | Gelatin, fibronectin and proteoglycan degradation, probably play a role in wound healing |
| MMP-13 | Collagen type I, II and III degradation, tissue remodelling |
| TIMP-1 | MMPs inhibition, cell proliferation promotion |
| TIMP-2 | MMPs inhibition, complements TIMP-1 in maintaining tissues hemostasis |
| RANKL | Bone remodelling and regeneration control, cell proliferation, with RANK binding promotes osteoclasts formation and maturation |
| Osteoprotegrin | Suppression of osteoclast formation by competitive binding to RANK |
5. Genetic Differences Increasing Risk of Peri-Implantitis
Genetic variability is an inherent factor in any species and can affect even the best-developed treatment for a disease. In peri-implantitis, some gene variations have implications for the susceptibility or acceleration of destruction in peri-implant tissues. The most commonly examined gene variations are genetic polymorphisms regarding IL-1α, IL-1β, TNA-α, MMP-8 and IL-10. The genetic polymorphisms are shown in Figure 5.
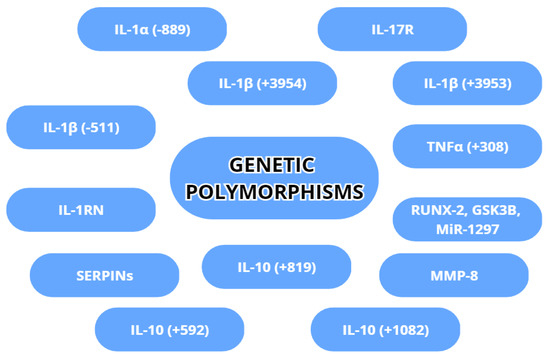
Figure 5. Genetic polymorphisms with a possible association with peri-implantitis.
6. Cellular Factors
The main cells present in the vicinity of implants are fibroblasts, neutrophils, osteocytes, macrophages and epithelial cells. Peri-implantitis is characterized by increased connective and pocket epithelial tissue levels [34]. Inflamed connective tissue shows high disorganization with great inflammatory cell infiltration (mainly plasma cells and neutrophils). Collagen matrix fibres often indicate a lack of accompanying fibroblasts, which show modulatory effects on inflammation processes and the reconstruction of collagen [35]. Instead, there is inflammatory destruction of the collagen skeleton. The prevalence of collagen types has shifted from collagen type I to collagen type III [36]. Changes noticed on microscopic cross-sections were greater than in periodontitis. An analysis of grafted peri-implantitis connective tissues showed the expression of CD68, MPO and iNOS surface proteins. On the other hand, 8-deoxyguanosine (8-OHdG) markers, both nuclear and mitochondrial, showed a decrease in peri-implantitis compared to periodontitis, indicating decreased heat-shock RNA destruction. In peri-implantitis epithelial cells, membranous protein expression showed differences in the levels of γ-H2AX, iNOS, NOX2, MPO and the PAD4/MPO ratio [34]. Interestingly, not every study detected changes between the control group and peri-implantitis in the peri-implant epithelium [1]. As for immunological cells, the area of inflammation in peri-implantitis consists of commonly found neutrophils, plasma cells, macrophages and T-cells. Neutrophils particularly densely infiltrate the coronal part of the implant, whereas deeper parts show a higher number of M1-type macrophages and a shift in the M1/M2 ratio [20][37][38]. The shift in the balance of M1/M2 macrophages can play a role in the osteolytic effect and progression. It has also been noted that while exposed to PDLF, macrophages reduced the excretion of TNFα significantly and of IL-1β minimally [39]. This behaviour of macrophages shows interesting directions in which we can modulate an inflammatory response [40]. A coexisting rise in the number of dendritic cells can also negatively affect Langerhans cell maturation and modulation of the intensity of inflammation [20]. The most important cells in peri-implantitis are listed with their function in Table 5.
Table 5. Cells present in peri-implantitis tissues and their functions.
| Cells Type | Function and Disfunction |
|---|---|
| Epithelial cells | Apical proliferation, γ-H2AX, iNOS, NOX2, MPO expression |
| Fibroblasts | Lowered collagen production, mainly type I and III |
| Macrophages | Tissue infiltration, cytokine production, phagocytosis |
| Neutrophils | Tissue infiltration, cytokine production, NETosis, ROS production |
| Osteocytes | Bone matrix production reduction, inability to repair the damages |
| Osteoclasts | Bone destruction, influences the bone metabolism |
| Plasma cells | Maintaining inflammation process, humoral immunity |
| T-type lymphocytes | Maintaining inflammation process, cellular immunity |
| Dendritic cells | Inflammation modulation, affects Langerhans cells response |
7. Diagnostic Opportunities (aMMP-8, TNFα, IL-1β, IL-6)
The spectrum of diagnostic molecules in peri-implantitis that is taken into consideration has broadened in recent years. MMP-8 clearly leads the way in peri-implantitis screening and even grading. One of the most often used tests in research is the point-of-care/chairside test. The measurements for peri-implantitis of aMMP-8 are <20 ng/mL = low risk, 20–80 ng/mL = elevated risk and >80 ng/mL = high risk. The screening of MMP-8 can help in the early stages of peri-implant mucositis and peri-implantitis, allowing for quick therapeutic intervention [41]. The level of MMP-8 decreases with correct treatment and can be used for treatment evaluation [12]. While using the point-of-care/chairside enzyme test, aMMP-8 has the highest sensitivity when compared with IL-6 [42][43][44]. However, it must be said that for MMP-8 (and IL-1β and IL-6, too), the PoC/chairside test is dependent on the PD of the diseased implant. Other studies compared ELISA and an Immunoassay for MMP-8 screening, with the Immunoassay performing better than ELISA [22]. MMP-8 is also a promising biomarker for peri-implant osteolysis around diseased implants [40]. Another important biomarker in diagnostics is IL-1β. IL-1β levels in peri-implantitis are elevated from the very beginning; hence, it can be used for diagnostic purposes before clinical manifestations [20][45][46][47][48]. It is detected in the same way as MMP-8, that is, using the PoC/chairside test [22]. Moreover, IL-1β levels positively correlate with lost implants [21]. Periodic clinical controls facilitate the detection of a transition to more severe peri-implant disease stages [49]. TNFα has similar diagnostic properties to IL-1β [21][46], but shows less specificity. TNFα is also tricky to classify, due to the difficulty in setting benchmark levels, as smaller TNFα concentrations are recognized in peri-implantitis than in medium-grade periodontitis [50]. Last, but not least, is IL-6. Thus far, it is the least used cytokine of those mentioned above in peri-implantitis diagnosis. It can be detected using the PoC/chairside test, but the results have a higher diagnostic bias compared to MMP-8 [42]. Nonetheless, it may provide sufficient data for peri-implant recognition, especially with a deep probing depth noted [45].
This entry is adapted from the peer-reviewed paper 10.3390/dj11050134
References
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S286–S291.
- Buser, D.; Weber, H.P.; Lang, N.P. Tissue integration of non-submerged implants. 1-year results of a prospective study with 100 ITI hollow-cylinder and hollow-screw implants. Clin. Oral Implant. Res. 1990, 1, 33–40.
- Misch, C.E.; Perel, M.L.; Wang, H.-L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P.; Steigmann, M.; Rebaudi, A.; Palti, A.; Pikos, M.A.; et al. Implant success, survival, and failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant. Dent. 2008, 17, 5–15.
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S1–S8.
- Araujo, M.G.; Lindhe, J. Peri-implant health. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S230–S236.
- Sahrmann, P.; Gilli, F.; Wiedemeier, D.B.; Attin, T.; Schmidlin, P.R.; Karygianni, L. The Microbiome of Peri-Implantitis: A Systematic Review and Meta-Analysis. Microorganisms 2020, 8, 661.
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172, Erratum in J. Periodontol. 2018, 89, 1475.
- Heitz-Mayfield, L.J.A.; Salvi, G.E. Peri-implant mucositis. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S237–S245.
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-implantitis. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S246–S266.
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S278–S285.
- Silva, R.C.E.; Reis, M.B.L.; Arid, J.; Flores, E.K.B.; Cruz, G.V.; Marañón-Vásquez, G.A.; De Souza, L.K.F.; Novaes, A.B., Jr.; De Queiroz, A.M.; Küchler, E.C. Association between Genetic Polymorphisms in RANK, RANKL and OPG and Peri-Implant Diseases in Patients from the Amazon Region. Braz. Dent. J. 2020, 31, 63–68.
- Thierbach, R.; Maier, K.; Sorsa, T.; Mäntylä, P. Peri-Implant Sulcus Fluid (PISF) Matrix Metalloproteinase (MMP)-8 Levels in Peri-Implantitis. J. Clin. Diagn. Res. 2016, 10, ZC34–ZC38.
- Astolfi, V.; Ríos-Carrasco, B.; Gil-Mur, F.J.; Ríos-Santos, J.V.; Bullón, B.; Herrero-Climent, M.; Bullón, P. IIncidence of Peri-Implantitis and Relationship with Different Conditions: A Retrospective Study. Int. J. Environ. Res. Public Health 2022, 19, 4147.
- Plemmenos, G.; Piperi, C. Pathogenic Molecular Mechanisms in Periodontitis and Peri-Implantitis: Role of Advanced Glycation End Products. Life 2022, 12, 218.
- Petkovic-Curcin, A.; Zeljic, K.; Cikota-Aleksic, B.; Dakovic, D.; Tatic, Z.; Magic, Z. Association of Cytokine Gene Polymorphism with Peri-implantitis Risk. Int. J. Oral Maxillofac. Implant. 2017, 32, e241–e248.
- Insua, A.; Monje, A.; Wang, H.L.; Miron, R.J. Basis of bone metabolism around dental implants during osseointegration and peri-implant bone loss. J. Biomed. Mater. Res. A 2017, 105, 2075–2089.
- D’Ambrosio, F.; Caggiano, M.; Schiavo, L.; Savarese, G.; Carpinelli, L.; Amato, A.; Iandolo, A. Chronic Stress and Depression in Periodontitis and Peri-Implantitis: A Narrative Review on Neurobiological, Neurobehavioral and Immune-Microbiome Interplays and Clinical Management Implications. Dent. J. 2022, 10, 49.
- Mancini, L.; Americo, L.M.; Pizzolante, T.; Donati, R.; Marchetti, E. Impact of COVID-19 on Periodontitis and Peri-Implantitis: A Narrative Review. Front. Oral Health 2022, 3, 822824.
- Sahoo, S.K.; Jalaluddin, M.; Bhuyan, L.; Dash, K.C.; Mishra, S.; Mishra, P. Assessment of Cytokine and Herpesvirus Level in Peri-implantitis and Healthy Patients. J. Pharm. Bioallied. Sci. 2021, 13 (Suppl. S2), S1418–S1421.
- Baseri, M.; Radmand, F.; Hamedi, R.; Yousefi, M.; Kafil, H.S. Immunological Aspects of Dental Implant Rejection. BioMed Res. Int. 2020, 2020, 7279509.
- Corrêa, M.G.; Pimentel, S.P.; Ribeiro, F.V.; Cirano, F.R.; Casati, M.Z. Host response and peri-implantitis. Braz. Oral. Res. 2019, 33 (Suppl. S1), e066.
- Al-Majid, A.; Alassiri, S.; Rathnayake, N.; Tervahartiala, T.; Gieselmann, D.R.; Sorsa, T. Matrix Metalloproteinase-8 as an Inflammatory and Prevention Biomarker in Periodontal and Peri-Implant Diseases. Int. J. Dent. 2018, 2018, 7891323.
- Nickles, K.; Krebs, M.; Schacher, B.; Petsos, H.; Eickholz, P. Long-Term Results after Placing Dental Implants in Patients with Papillon-Lefèvre Syndrome: Results 2.5–20 Years after Implant Insertion. J. Clin. Med. 2022, 11, 2438.
- Al-Askar, M.; Ajlan, S.; Alomar, N.; Al-Daghri, N.M. Clinical and Radiographic Peri-Implant Parameters and Whole Salivary Interleukin-1β and Interleukin-6 Levels among Type-2 Diabetic and Nondiabetic Patients with and without Peri-Implantitis. Med. Princ. Pract. 2018, 27, 133–138.
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433.
- Naruishi, K.; Nagata, T. Biological effects of interleukin-6 on Gingival Fibroblasts: Cytokine regulation in periodontitis. J. Cell. Physiol. 2018, 233, 6393–6400.
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806.
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486.
- Groeger, S.; Meyle, J. Oral Mucosal Epithelial Cells. Front. Immunol. 2019, 10, 208.
- Abusleme, L.; Moutsopoulos, N.M. IL-17: Overview and role in oral immunity and microbiome. Oral Dis. 2017, 23, 854–865.
- Bunte, K.; Beikler, T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 3394.
- Wang, P.-L.; Shirasu, S.; Shinohar, M.; Azuma, Y.; Daito, M.; Yasuda, H.; Ohura, K. IL-10 inhibits Porphyromonas gingivalis LPS-stimulated human gingival fibroblasts production of IL-6. Biochem. Biophys. Res. Commun. 1999, 263, 372–377.
- Gettins, P.G.; Olson, S.T. Inhibitory serpins. New insights into their folding, polymerization, regulation and clearance. Biochem. J. 2016, 473, 2273–2293.
- Dionigi, C.; Larsson, L.; Carcuac, O.; Berglundh, T. Cellular expression of DNA damage/repair and reactive oxygen/nitrogen species in human periodontitis and peri-implantitis lesions. J. Clin. Periodontol. 2020, 47, 1466–1475.
- Fernandes, M.H.; Gomes, P.S. Bone Cells Dynamics during Peri-Implantitis: A Theoretical Analysis. J. Oral Maxillofac. Res. 2016, 7, e6.
- Mijiritsky, E.; Ferroni, L.; Gardin, C.; Peleg, O.; Gultekin, A.; Saglanmak, A.; Delogu, L.G.; Mitrecic, D.; Piattelli, A.; Tatullo, M.; et al. Presence of ROS in Inflammatory Environment of Peri-Implantitis Tissue: In Vitro and In Vivo Human Evidence. J. Clin. Med. 2019, 9, 38.
- Li, Y.; Ling, J.; Jiang, Q. Inflammasomes in Alveolar Bone Loss. Front. Immunol. 2021, 12, 691013.
- Galarraga-Vinueza, M.E.; Obreja, K.; Ramanauskaite, A.; Magini, R.; Begic, A.; Sader, R.; Schwarz, F. Macrophage polarization in peri-implantitis lesions. Clin. Oral Investig. 2021, 25, 2335–2344.
- Tzach-Nahman, R.; Nashef, R.; Fleissig, O.; Palmon, A.; Shapira, L.; Wilensky, A.; Nussbaum, G. Oral fibroblasts modulate the macrophage response to bacterial challenge. Sci. Rep. 2017, 7, 11516.
- Alassy, H.; Parachuru, P.; Wolff, L. Peri-Implantitis Diagnosis and Prognosis Using Biomarkers in Peri-Implant Crevicular Fluid: A Narrative Review. Diagnostics 2019, 9, 214.
- Aleksandrowicz, P.; Żelechowska, P.; Agier, J.; Starska, K.; Kędzierski, K.; Wysokińska-Miszczuk, J.; Brzezińska-Błaszczyk, E. Evaluation of Metalloproteinase-8 Levels in Crevicular Fluid of Patients with Healthy Implants or Periodontitis. Mediat. Inflamm. 2017, 2017, 4920847.
- Lähteenmäki, H.; Tervahartiala, T.; Räisänen, I.T.; Pärnänen, P.; Mauramo, M.; Gupta, S.; Sampson, V.; Rathnayake, N.; Heikkinen, A.; Alassiri, S.; et al. Active MMP-8 point-of-care (PoC)/chairside enzyme-test as an adjunctive tool for early and real-time diagnosis of peri-implantitis. Clin. Exp. Dent. Res. 2022, 8, 485–496.
- Farhad, S.Z.; Rezazadeh, F.; Mohammadi, M. Interleukin-17 and Interleukin-10 as Inflammatory and Prevention Biomarkers in Periimplant Diseases. Int. J. Prev. Med. 2019, 10, 137.
- Guarnieri, R.; Zanza, A.; D’Angelo, M.; Di Nardo, D.; Del Giudice, A.; Mazzoni, A.; Reda, R.; Testarelli, L. Correlation between Peri-Implant Marginal Bone Loss Progression and Peri-Implant Sulcular Fluid Levels of Metalloproteinase-8. J. Pers. Med. 2022, 12, 58.
- Ghassib, I.; Chen, Z.; Zhu, J.; Wang, H.L. Use of IL-1 β, IL-6, TNF-α, and MMP-8 biomarkers to distinguish peri-implant diseases: A systematic review and meta-analysis. Clin. Implant. Dent. Relat. Res. 2019, 21, 190–207.
- Zhang, X.; Wang, Z.; Hu, L.; Shen, X.; Liu, C. Identification of Potential Genetic Biomarkers and Target Genes of Peri-Implantitis Using Bioinformatics Tools. BioMed Res. Int. 2021, 2021, 1759214.
- García-Delaney, C.; Sánchez-Garcés, M.Á.; Figueiredo, R.; Sánchez-Torres, A.; Gay-Escoda, C. Clinical significance of interleukin-1 genotype in smoking patients as a predictor of peri-implantitis: A case-control study. Med. Oral Patol. Oral Cir. Bucal. 2015, 20, e737–e743.
- Kormas, I.; Pedercini, C.; Pedercini, A.; Raptopoulos, M.; Alassy, H.; Wolff, L.F. Peri-Implant Diseases: Diagnosis, Clinical, Histological, Microbiological Characteristics and Treatment Strategies. A Narrative Review. Antibiotics 2020, 9, 835.
- Gleiznys, D.; Gleiznys, A.; Abraškevičiūtė, L.; Vitkauskienė, A.; Šaferis, V.; Sakalauskienė, J. Interleukin-10 and Interleukin-1β Cytokines Expression in Leukocytes of Patients with Chronic Peri-Mucositis. Med. Sci. Monit. 2019, 25, 7471–7479.
- Aleksandrowicz, P.; Brzezińska-Błaszczyk, E.; Kozłowska, E.; Żelechowska, P.; Borgonovo, A.E.; Agier, J. Analysis of IL-1β, CXCL8, and TNF-α levels in the crevicular fluid of patients with periodontitis or healthy implants. BMC Oral Health 2021, 21, 120.
This entry is offline, you can click here to edit this entry!
