Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Chemical
Forward osmosis (FO) has attracted special attention in water and wastewater treatment due to its role in addressing the challenges of water scarcity and contamination. The presence of emerging contaminants in water sources raises concerns regarding their environmental and public health impacts. Conventional wastewater treatment methods cannot effectively remove these contaminants; thus, innovative approaches are required. FO membranes offer a promising solution for wastewater treatment and removal of the contaminants in wastewater.
- municipal wastewater
- contaminants
- membranes
1. Introduction
Water scarcity and contamination are considered serious problems of worldwide concern, in relation to both industrial requirements and population growth [1,2]. In addition to current water scarcity, it is estimated that water shortage could increase up to 60% by 2025 [3,4]. The sixth sustainable development goal of the 2030 agenda focuses on the availability and sustainable management of water and sanitation for all.
Therefore, an efficient management of water resources is necessary. In the prosecution of this aim, wastewater treatment plants (WWTPs) play a fundamental role. It should be noted that municipal WWTPs are designed to reduce pollution and to protect environmental quality and human health, in addition to obtaining benefits such as water, nutrients, and energy [5,6].
WWTPs are facilities that treat the wastewaters (WW) generated by an area or city; therefore, an increase in urban population directly influences WW discharges that must be controlled and treated so that they do not pose a risk to humans and the environment.
Increasing environmental constraints worldwide are creating the need to adapt conventional wastewater plants to more sustainable and robust treatment systems, employing new treatment technologies and combining low environmental impact and energy efficiency [7,8]. The design of sustainable wastewater treatment systems must focus on environmental protection, while minimizing energy and resource consumption [9]. Conventional wastewater treatment typically consists of a combination of physical, chemical, and biological processes and operations in order to remove solids, organic matter, and sometimes, nutrients from wastewater [10]. The physical processes include screening, sedimentation, and filtration, while the chemical processes include coagulation, flocculation, and disinfection. The biological processes involve the use of microorganisms to break down organic matter and nutrients in wastewater [11]. The combination of these processes and operations can effectively treat wastewater and reduce its potential impact on the environment and human health. However, conventional wastewater treatment plants cannot efficiently remove emerging pollutants such as drugs, hormones, and pesticides. Thus, many efforts have been made to develop effective technologies for wastewater treatment over the past few decades aimed at removing pollutants from wastewater and providing nontoxic but ecofriendly processes [12]. Different advanced wastewater treatment technologies, such as membrane filtration, adsorption, and advanced oxidation processes are being investigated to improve the removal efficiency of emerging pollutants and nutrients [13]. It is important to consider the advantages and disadvantages of different treatment technologies and their effectiveness in removing pollutants from wastewater when selecting a treatment process. Membrane technology has emerged as a favorite choice for reclaiming water from different wastewater streams for reuse [14]. The integration of resource recovery in wastewater treatment plants can also contribute to environmental sustainability by reducing waste and producing valuable resources [15].
2. Problems in Wastewater Treatment
WWTPs include different levels of treatment, starting with a primary treatment where part of the organic matter and suspended solids are removed, followed by a secondary treatment to eliminate biodegradable organic matter and nutrients, and, in some cases, ending with a tertiary treatment or advanced wastewater treatment to remove suspended solids and disinfect water [16]. However, many developing countries do not have complete wastewater treatment plants or only include primary (physical treatment) and secondary (biological treatment) stages without any tertiary treatment or advanced sludge processing [17]. In addition, inadequate WWTP design and operation can cause serious environmental problems both locally and globally [18].
Currently, the conventional activated sludge (CAS) processes are the most common treatments in WWTPs [19]. These treatments involve a large amount of energy due to the high electrical demand for aeration; on the other hand, the cost increases due to the necessary treatment of the resulting sludge [20,21]. In addition, in this aerobic treatment of activated sludge, the carbon content of the wastewater is not effectively utilized, resulting in its conversion into biomass and carbon dioxide without being fully exploited [22].
For energy and nutrient recovery from wastewater, anaerobic digestion is a promising treatment [23]. In such treatment, less sludge is generated, and less energy is consumed. In addition, anaerobic treatment is in line with the assumption of a circular economy, takes advantage of the organic matter content present in urban wastewater to produce biogas (i.e., a renewable energy source), and reduces CO2 emissions, compared to aerobic treatment [24].
However, despite the advantages referred to above, there are some difficulties in the application of anaerobic digestion for direct wastewater treatment. One of the difficulties is the low organic load of the wastewater, which causes a significant increase in digester heating per unit of biogas production and, therefore, directly influences the economic viability of the process [25,26,27,28]. Nevertheless, the limitations of anaerobic wastewater treatment can be overcome with processes that pre-concentrate the organic content and nutrients of the wastewater, thus turning cost-effective anaerobic treatment into biogas production and nutrient recovery [25,27,28,29,30,31].
This requires new developments and technologies to establish more energy efficient systems on water treatment and reuse, with membrane technology being a promising alternative [32,33].
3. Forward Osmosis Development
3.1. Background of FO
FO, as an alternative membrane process in wastewater treatment, has attracted increasing interest in recent years. FO is the process in which water molecules pass through a semipermeable membrane, which separates two solutions, as shown in Figure 1. This transport and movement of molecules takes place due to the osmotic pressure difference (Δπ) which is the driving force in this phenomenon, as opposed to pressure-driven membrane processes. Thus, water is permeated passing through the membrane from the lowest solution concentration, FS, to the highest solute concentration solution, DS, while other solutes molecules are rejected [19,43]. FO has been investigated in various applications, such as seawater desalination [43], power generation [44], food processing [45], and wastewater treatment [46,47].
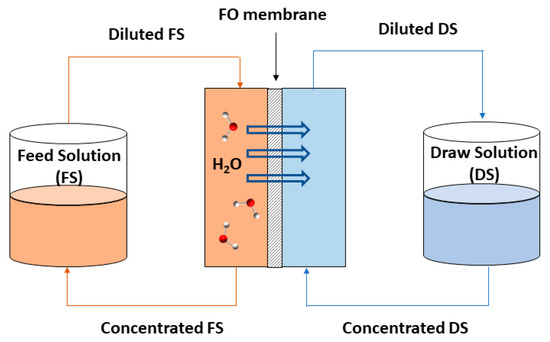
Figure 1. Scheme of the FO process.
The beginning of the interest in FO dates back to the 18th century [48,49], while interest in this field has increased due to the commercialization of membranes designed for this process [2]. Figure 2 shows the rising interest in membranes of FO in the last 20 years by analyzing the number of publications on the topic.
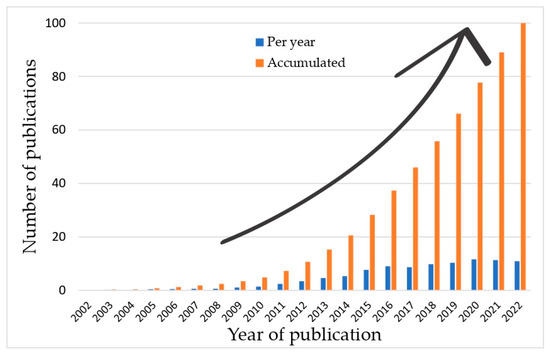
Figure 2. Yearly and accumulated numbers of publications on FO membranes (database: Scopus; search parameters: “forward osmosis membrane” in title, abstract, and keywords).
3.2. Types of FO Membranes
Forward osmosis membranes are of interest if they have elevated water permeability while keeping salt retention high. In addition, they must present low concentration polarization, which is a phenomenon that, in the forward osmosis process, causes the osmotic pressure to decrease, leading to a reduction in the flow of water through the membrane. Furthermore, good chemical and mechanical stability to withstand working conditions is required [50].
FO membrane modules can be classified into plate and frame, spiral wound, tubular, hollow fiber, and flat sheet, according to the various geometric structures. The most used FO membrane modules are flat sheets and especially hollow fibers because these configurations require little space and are capable of separating large volumes, which are advantageous factors when compared with other membrane module configurations [51].
The most common FO commercial membranes, with respect to the material used, are cellulose acetate/triacetate (CA/CTA)-based membrane and thin-film composite (TFC) membranes of polyamide, polysulfone, or polyester layers [52,53,54,55,56,57]. A recent study proposed a classification of the emerging FO membranes into four categories according to their fabrication methods: cellulose acetate (CA), thin-film composites (TFCs), polybenzimidazole (PBI), and aquaporin (AQP), with TFCs the most competitive according to their properties [58].
The first commercialized FO membranes, i.e., CA/CTA membranes, have advantages such as good mechanical resistance, low tendency to fouling, good permeate fluxes, and high resistance to chlorine [59]. However, the operation pH range (3–8) is somewhat limited. To improve the characteristics of CA/CTA membranes, TFC membranes with a pH range of 2–11 and with higher permeate fluxes have been produced [2,51].
In addition to commercial membranes, numerous recent studies tried to modify the structure of the support layer using different methods or additives such as silica, graphene, zeolite, and TiO2 to improve the properties of commercial membranes [51,60,61,62,63].
3.3. Main Manufacturers of FO Modules
Various industrial companies offer FO membranes and commercial FO systems. Initially, the pioneering company for the supply of FO membranes was Hydration Technology Innovations (HTI) founded in 1986 in Albany (NY, USA). Later, another company called Oasys Water Inc. began to commercialize FO modules in the year 2010 in Cambridge (MA, USA). Another firm that manufactures FO membranes is FTS H2O™, also working in Albany (USA), specializing in CTA membranes in flat sheets. Next, the company Aquaporin Inside™ introduced FO membranes with aquaporin proteins that are highly selective, facilitating the transport of water molecules. These thin-film composite membranes are available in both flat sheet and hollow fiber configurations. In addition, Aquaporin A/S, a developer of these biomimetic membranes based in Lyngby (Denmark), recently signed a development agreement with another leading tubular membrane manufacturing company called Berghof Membrane Technology based in Leeuwarden (the Netherlands) to launch new membranes. Other companies have manufactured or have collaborated in the manufacture of FO modules such as Toray, Toyobo, Koch membrane systems, and Porifera, as well as some intermediary companies for marketing this type of module such as Sterlitech [64]. It should be noted that the supply of this type of FO membranes has facilitated studies and research related to FO that otherwise would have been much less developed today.
3.4. Important Factors
The operating conditions significantly affect the performance of FO. Therefore, their optimization is necessary to make the FO process more efficient. For example, it is necessary to optimize the concentration of DS and FS, the flow rates of FS and DS, the pH, the temperature, and the orientation of the membrane, which can be the active layer facing FS (AL-FS) or active layer facing DS (AL-DS). Furthermore, it is important to control the characteristics and properties of the membrane such as material, mechanical and chemical stability, active area, porosity, and hydrophobicity [65].
In addition to the above, there are other relevant factors influencing the FO process that must be considered to solve possible drawbacks. Despite the wide variety of FO applications and the extensive FO-related research, there are some process issues and challenges that require still special attention for the process to maximize its commercial and industrial possibilities. These include the choice of the draw solution, the reduction in reverse salt flow, the regeneration of DS, and the reduction in concentration polarization and membrane fouling, as shown in Figure 3 [46].
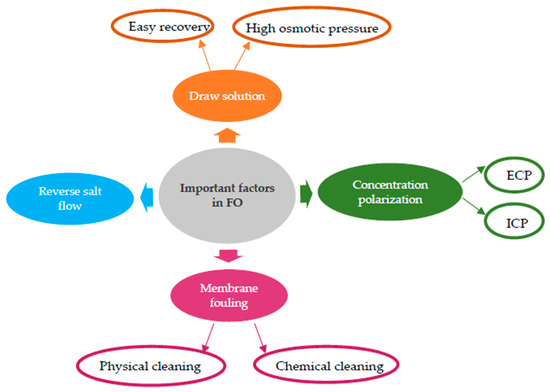
Figure 3. Important factors influencing the FO process.
Draw Solution
To choose the possible draw solutions, it must be taken into account that they should meet a series of characteristics and requirements. Some important qualities are that it must generate high osmotic pressures [43,66], be economic, safe, and nontoxic, give minimal reverse draw solution flux, be stable, not react with the membrane material, and be easy to recover [67]. Commonly, solutes with a high solubility in water are selected to avoid their diffusion through the membrane. To improve the performance of the membrane by reducing concentration polarization on the surface of both sides, it is favorable to choose solutes with small molecular weight, giving low viscosity in the aqueous solution. Another important criterion, from an energetic point of view, is to have an easy and/or useful recovery or regeneration [67]. Extractive solutions with very varied solutes (inorganic salts, volatile compounds, organic solutes, etc.) have been suggested and studied. To date, most inorganic salt solutions as NaCl, MgCl2, KNO3, and MgSO4 have been tested due to their low cost and high osmotic pressure, with sodium chloride (NaCl) frequently selected as a reference DS for several reasons. First, it is generally used for standard membrane tests allowing a comparison of the results obtained with data from the literature because NaCl is commonly used as a DS. Furthermore, seawater and reverse osmosis concentrate are widely used as DSs in several interesting applications [68]. However, there are other interesting potential inorganic DSs depending on their characteristics and applications. For example, K4P2O7, KCl, and NH4PO3, which have the advantage of having fertilizing properties and providing high osmotic pressure, can be used as DSs if the end use of the water recovered is in irrigation. In this case, DS recovery would not be necessary [69,70], with subsequent economic savings. Organic-based solutes, compared to inorganic solutes, tend to have higher molecular weights, making their utilization somewhat more challenging. These solutes typically include sugars, diethyl ether, or organic salts. Studies have been conducted using common food additives such as monosodium glutamate (MSG), saccharin (SAS), and trisodium citrate (TSC), which generate slightly higher osmotic pressures but lower water flux than NaCl [71].
In addition, in some processes, gases such as CO2, SO2, and NH3 have been used due to their good solubility in water. However, they have not been implemented in real processes due to their limited osmotic pressure and high energy consumption requirements. There are also other less developed proposals for using magnetic solutes and hydrogels, which currently make the processes more expensive and are not sufficiently understood [72,73].
At present, the choice of DS and its regeneration are key issues in the application of FO. Energy-consuming solute recovery is one of the major considerations in selecting the DS. Some regeneration methods may consist of their direct use without ulterior recovery [74]. In some cases, DS is regenerated by membrane separation, such as RO [75], NF [76], UF [77], MD [78], ED [79], chemical precipitation [80], or thermal separation [81]. Other options are magnetic recovery and electrolytic recovery. Although there are various methods for DS regeneration, each method has its advantages and limitations for the application of the FO process [51].
This entry is adapted from the peer-reviewed paper 10.3390/membranes13070655
This entry is offline, you can click here to edit this entry!
