Early onset colorectal cancer (EOCRC) is defined as CRC diagnosed in individuals younger than 50, which is generally considered the ideal age to start screening programs in the average-risk population. Although the overall incidence of colorectal cancer (CRC) is declining, the number of new diagnoses in patients younger than 50 is alarmingly increasing.
- colorectal cancer
- early onset
- screening
1. Introduction
Early onset colorectal cancer (EOCRC) is defined as CRC diagnosed in individuals younger than 50, which is generally considered the ideal age to start screening programs in the average-risk population. Although the overall incidence of colorectal cancer (CRC) is declining, the number of new diagnoses in patients younger than 50 is alarmingly in- creasing [1][2]. Modifiable and nonmodifiable factors, such as antibiotic exposure, obesity, a Western diet, diabetes mellitus, inflammatory bowel disease (IBD), environmental pollution,
and pesticide use, might be among the possible causes [3]. However, the exact reasons for this rising phenomenon are still unknown.
EOCRC seems to have different features than CRC in older patients. EOCRC generally develops with more aggressive features, is diagnosed at a more advanced stage [4][5][6][7], and has stronger metastatic potential [8]. On the other hand, young people with metastatic cancer have better overall survival (OS), probably related to better performance status, lower comorbidities, higher tolerance to chemotherapy treatments, and lower postsurgical mortality [9].
Of note, the advanced stage at diagnosis might be related to the fact that screening campaigns do not involve the population under the age of 50, except among individuals with a family history of CRC or those affected by chronic IBD [3][10]. The noteworthy increase in CRC cases among young people in the last decade might lead to considering the need to lower the age of starting screening; however, these measures would result in increased healthcare costs.
Given the greater proportion of young patients that present with advanced disease at the time of diagnosis, young adults with Stage IV EOCRC represent a small but increasing and relevant population [11] that needs greater attention and further study. For this reason, we herein present a comprehensive review of the literature on EOCRC, with a particular focus on metastatic disease.
2. Epidemiology
CRC is the third most common malignancy and cause of cancer death worldwide in both genders, mostly over the age of 50 [12]. Since the mid-1980s, incidence and mortality have each decreased, likely due to both the start of screening programs and the optimization of disease management [13]. However, this progress is confined to older individuals, and multiple studies have revealed an alarming increasing incidence among people younger than 50 [10][14][15][16]. Namely, in the last twenty years, the median age of CRC diagnosis has decreased from 72 to 66. In addition, 10 to 20% of CRC diagnoses involve people younger than 50, and about three-quarters of them are aged 40–49 (SEER Stat Database) [17]. Of note, the increase in EOCRC IRs has been mainly driven by rectal cancer diagnoses [18], which have risen by more than 90% from the beginning of the 1990s to 2016 (from 2.6 to 5.1/100,000) compared to an increase of about 40% for colon cancer [17]. With regard to sex differences, whereas the incidence of CRC in the 55–74 age group is almost 50% greater in men than in women, it is comparable between men and women diagnosed earlier than at 40 [19][20].
Considering the current data and despite general trends toward population aging, a retrospective cohort study foresees by 2030 an increase in colon cancer diagnosis of 90% in the 20–34 age cohort and 27.7% in the 35–49 age cohort, with an even higher rise for rectal cancer diagnosis of 124% and 46% for the two subgroups, respectively [21][22].
- Geographic Differences
As displayed in Figures 1 and 2, global EOCRC incident rates (IRs) fluctuate from
3.5 per 100,000 inhabitants in India to 12.9 in the Republic of Korea [23]. In the last decade, an increasing IR was recorded in 19 out of 36 countries, among which 9 (e.g., Australia, Germany, and the US) had stable or declining trends in older adults. Only three countries (Austria, Italy, and Lithuania) exhibited a decrease in EOCRC IRs [18]. A similar distribution emerged from a further recent study [24], which also showed how the increase is mainly attributable to rectal cancer, with the exception of the United Kingdom and Brazil. The highest incidence of EOCRC was found in females in Switzerland (4.2/100,000) and in males in the Republic of Korea (4.6/100,000), with no difference in trend variation between rectal and colon cancer [25].
In the United States (US), age-specific CRC risk has returned to the levels recorded in those born around 1890 [15]. Here, an increase in colon cancer IRs was seen both in the 20–39 age cohort (from 1% to 2.4% annually since the mid-1980s) and in the 40–54 age cohort (from 0.5% to 1.3% annually since the mid-1990s). A faster increase in rectal cancer IRs (3.2% annually from 1974 to 2013 in adults aged 20–29 years) was also reported in the US, where from 1990 to 2013, the number of diagnoses in the under-55 population doubled from 14.6% to 29.2% [15]. It is noteworthy that in the US, ethnic differences have been reported; in particular, non-Hispanic black individuals have been reported to be at a higher risk of EOCRC development [22][23][24][25][26][27], especially in rural areas [28].
A similar trend was also observed in European countries, where from 2004 to 2016, CRC IRs increased by 7.9% annually in the age group of 20–29 years, 4.9% among those aged 30–39 years, and 1.6% in the 40–49 cohort [29].
With regard to non-Western populations, in recent years, the incidence of EOCRC has also been increasing in Arabic countries, which could be attributed to improved diagnostic strategies and changes in lifestyle and dietary habits, which have become more similar to those of Western countries [30]. An IR increase has also been reported in Iran and Egypt [19]. The age at CRC diagnosis in Africa and Asia is lower than in Europe and America [31], probably due to heritable causes, although no causes can be found in the literature to explain a different epidemiological trend from the Western population.
When talking about geographic differences, an important factor to consider is the presence of a private healthcare system, as EOCRC seems more prevalent among patients with no insurance coverage or ready access to care (16.5% vs. 4.7%), often of nonwhite ethnicity (29.5% vs. 17.6%) [7].
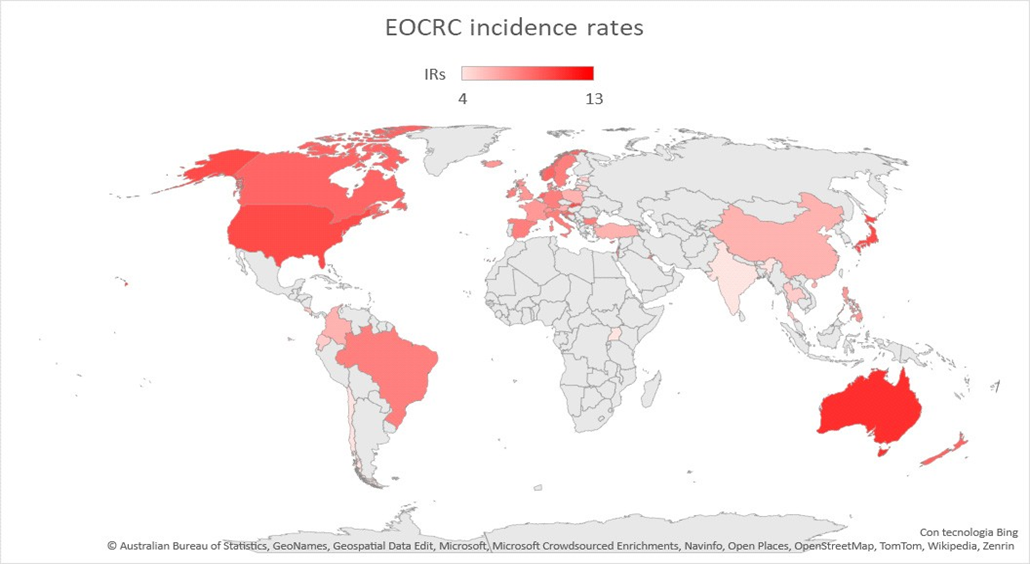
Figure 1. Map showing EOCRC incidence rates worldwide. Red countries are those in which an increased incidence rate of EOCRC has been documented [18][19][20][31][32].
- Screening Programs
Considering that most EOCRC diagnoses are made in people with an average screen- ing risk, and almost half of these patients are aged between 45 and 49 (SEER-Stat Database), since 2018, the American Cancer Society has indicated 45 as the optimal age to initiate CRC screening [33].
Screening in individuals younger than 50 is universally recommended for people with an elevated risk of CRC because of chronic IBD, familial syndromes, or with a family history of CRC in a first-degree relative (FDR) [10]. In these cases, screening colonoscopy is indicated by the age of 40 or, for some international societies (e.g., the American College of Gastroenterology, U.S. Multi-Society Task Force of Colorectal Cancer), 10 years prior to the age of diagnosis of advanced adenoma in the FDR before the age of 60, with a follow-up colonoscopy every 5 years. Other high-risk groups that received conditional recommendations to initiate early screening include African American individuals [34], cystic fibrosis patients [32], and people who underwent pelvic radiation (>30 Gy) at a young age [35].
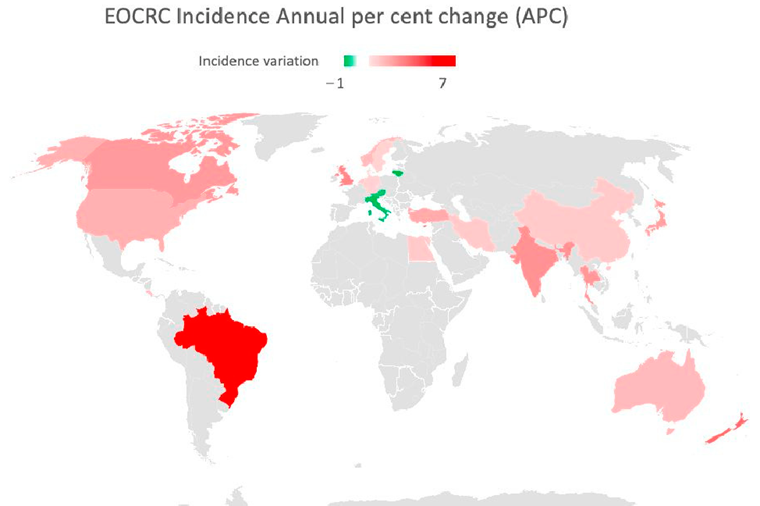
Figure 2. Map showing EOCRC incidence annual per cent change (APC) in the last 30 years. Red countries are those in which an increased APC has been documented. Green countries have experi- enced a decrease in APC [18][19][20][31][32].
This entry is adapted from the peer-reviewed paper 10.3390/cancers15133509
References
- 1. Saad El Din, K.; Loree, J.M.; Sayre, E.C.; Gill, S.; Brown, C.J.; Dau, H.; De Vera, M.A. Trends in the epidemiology of young-onset colorectal cancer: A worldwide systematic review. BMC Cancer 2020, 20, 288. https://doi.org/10.1186/s12885-020-06766-9.2. Nguyen, L.H.; Liu, P.-H.; Zheng, X.; Keum, N.; Zong, X.; Li, X.; Wu, K.; Fuchs, C.S.; Ogino, S.; Ng, K.; et al. Sedentary Be-haviors, TV Viewing Time, and Risk of Young-Onset Colorectal Cancer. JNCI Cancer Spectr. 2018, 2, pky073. https://doi.org/10.1093/jncics/pky073.
- Nguyen, L.H.; Liu, P.-H.; Zheng, X.; Keum, N.; Zong, X.; Li, X.; Wu, K.; Fuchs, C.S.; Ogino, S.; Ng, K.; et al. Sedentary Be-haviors, TV Viewing Time, and Risk of Young-Onset Colorectal Cancer. JNCI Cancer Spectr. 2018, 2, pky073. https://doi.org/10.1093/jncics/pky073.
- Gausman, V.; Dornblaser, D.; Anand, S.; Hayes, R.B.; O’Connell, K.; Du, M.; Liang, P.S. Risk Factors Associated With Early-Onset Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 2752–2759.e2. https://doi.org/10.1016/j.cgh.2019.10.009.
- Uy, G.B.; Kaw, L.L.; Punzalan, C.K.; Querol, R.I.L.C.; Koustova, E.V.; Bowyer, M.W.; Hobbs, C.M.; Sobin, L.H.; Wherry, D.C. Clinical and molecular biologic characteristics of early-onset versus late-onset colorectal carcinoma in Filipinos. World J. Surg. 2004, 28, 117–123. https://doi.org/10.1007/s00268-003-7281-4.
- Silla, I.O. Early-onset colorectal cancer: A separate subset of colorectal cancer. WJG 2014, 20, 17288. https://doi.org/10.3748/wjg.v20.i46.17288.
- Dozois, E.J.; Boardman, L.A.; Suwanthanma, W.; Limburg, P.J.; Cima, R.R.; Bakken, J.L.; Vierkant, R.A.; Aakre, J.A.; Larson, D.W. Young-Onset Colorectal Cancer in Patients With No Known Genetic Predisposition: Can We Increase Early Recognition and Improve Outcome? Medicine 2008, 87, 259–263. https://doi.org/10.1097/MD.0b013e3181881354.
- You, Y.N.; Xing, Y.; Feig, B.W.; Chang, G.J.; Cormier, J.N. Young-Onset Colorectal Cancer: Is It Time to Pay Attention? Arch. Intern. Med. 2012, 172, 287–289.
- Willauer, A.N.; Liu, Y.; Pereira, A.A.L.; Lam, M.; Morris, J.S.; Raghav, K.P.S.; Morris, V.K.; Menter, D.; Broaddus, R.; Mer-ic‐Bernstam, F.; et al. Clinical and molecular characterization of early‐onset colorectal cancer. Cancer 2019, 125, 2002–2010. https://doi.org/10.1002/cncr.31994.
- Liang, J.T.; Huang, K.C.; Cheng, A.L.; Jeng, Y.M.; Wu, M.S.; Wang, S.M. Clinicopathological and molecular biological fea-tures of colorectal cancer in patients less than 40 years of age. Br. J. Surg. 2003, 90, 205–214. https://doi.org/10.1002/bjs.4015.
- Siegel, R.L.; Jakubowski, C.D.; Fedewa, S.A.; Davis, A.; Azad, N.S. Colorectal Cancer in the Young: Epidemiology, Preven-tion, Management. Am. Soc. Clin. Oncol. Educ. Book 2020, e75–e88. https://doi.org/10.1200/EDBK_279901.
- Van Der Heide, D.M.; Turaga, K.K.; Chan, C.H.F.; Sherman, S.K. Mismatch Repair Status Correlates with Survival in Young Adults with Metastatic Colorectal Cancer. J. Surg. Res. 2021, 266, 104–112. https://doi.org/10.1016/j.jss.2021.03.040.
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J Clin. 2023, 73, 17–48. https://doi.org/10.3322/caac.21763.
- 13. Edwards, B.K.; Ward, E.; Kohler, B.A.; Eheman, C.; Zauber, A.G.; Anderson, R.N.; Jemal, A.; Schymura, M.J.; Lansdorp-Vogelaar, I.; Seeff, L.C.; et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010, 116, 544–573. https://doi.org/10.1002/cncr.24760.
- 14. Siegel, R.L.; Miller, K.D.; Jemal, A. Colorectal Cancer Mortality Rates in Adults Aged 20 to 54 Years in the United States, 1970–2014. JAMA 2017, 318, 572. https://doi.org/10.1001/jama.2017.7630.
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Pat-terns in the United States, 1974–2013. JNCI J. Natl. Cancer Inst. 2017, 109. https://doi.org/10.1093/jnci/djw322.
- Venugopal, A.; Carethers, J.M. Epidemiology and biology of early onset colorectal cancer. EXCLI J. 2022, 21, 162, ISSN 1611-2156. https://doi.org/10.17179/EXCLI2021-4456.
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017: Colorectal Cancer Statistics, 2017. CA A Cancer J. Clin. 2017, 67, 177–193. https://doi.org/10.3322/caac.21395.
- Murphy, C.C.; Wallace, K.; Sandler, R.S.; Baron, J.A. Racial Disparities in Incidence of Young-Onset Colorectal Cancer and Patient Survival. Gastroenterology 2019, 156, 958–965. https://doi.org/10.1053/j.gastro.2018.11.060.
- Abou-Zeid, A.A.; Jumuah, W.A.; Ebied, E.F.; Abd El Samee Atia, K.S.; El Ghamrini, Y.; Somaie, D.A. Hereditary factors are unlikely behind unusual pattern of early—Onset colorectal cancer in Egyptians: A study of family history and pathology fea-tures in Egyptians with large bowel cancer (cross-sectional study). Int. J. Surg. 2017, 44, 71–75. https://doi.org/10.1016/j.ijsu.2017.06.028.
- Mauri, G.; Sartore‐Bianchi, A.; Russo, A.; Marsoni, S.; Bardelli, A.; Siena, S. Early‐onset colorectal cancer in young individuals. Mol. Oncol. 2019, 13, 109–131. https://doi.org/10.1002/1878-0261.12417.
- Bailey, C.E.; Hu, C.-Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing Disparities in the Age-Related Incidences of Colon and Rectal Cancers in the United States, 1975-2010. JAMA Surg 2015, 150, 17. https://doi.org/10.1001/jamasurg.2014.1756.
- Stoffel, E.M.; Murphy, C.C. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology 2020, 158, 341–353. https://doi.org/10.1053/j.gastro.2019.07.055.
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorec-tal cancer incidence in young adults. Gut 2019, 68, 2179–2185. https://doi.org/10.1136/gutjnl-2019-319511.
- Lui, R.N.; Tsoi, K.K.F.; Ho, J.M.W.; Lo, C.M.; Chan, F.C.H.; Kyaw, M.H.; Sung, J.J.Y. Global Increasing Incidence of Young-Onset Colorectal Cancer Across 5 Continents: A Joinpoint Regression Analysis of 1,922,167 Cases. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1275–1282. https://doi.org/10.1158/1055-9965.EPI-18-1111.
- Lu, X.; Li, Y.; Wang, W.; Feng, W.; Shi, O.; Wang, Q. International incidence trends in early- and late-onset colorectal cancer: A population-based study. Int J Color. Dis 2020, 35, 1077–1086. https://doi.org/10.1007/s00384-020-03558-2.
- Yeo, H.; Betel, D.; Abelson, J.S.; Zheng, X.E.; Yantiss, R.; Shah, M.A. Early-onset Colorectal Cancer is Distinct From Tradi-tional Colorectal Cancer. Clin. Color. Cancer 2017, 16, 293-299.e6. https://doi.org/10.1016/j.clcc.2017.06.002.
- Theuer, C.P.; Wagner, J.L.; Taylor, T.H.; Brewster, W.R.; Tran, D.; McLaren, C.E.; Anton–Culver, H. Racial and ethnic colo-rectal cancer patterns affect the cost-effectiveness of colorectal cancer screening in the United States. Gastroenterology 2001, 120, 848–856. https://doi.org/10.1053/gast.2001.22535.
- Zahnd, W.E.; Gomez, S.L.; Steck, S.E.; Brown, M.J.; Ganai, S.; Zhang, J.; Arp Adams, S.; Berger, F.G.; Eberth, J.M. Ru-ral‐urban and racial/ethnic trends and disparities in early‐onset and average‐onset colorectal cancer. Cancer 2021, 127, 239–248. https://doi.org/10.1002/cncr.33256.
- Ahnen, D.J.; Wade, S.W.; Jones, W.F.; Sifri, R.; Mendoza Silveiras, J.; Greenamyer, J.; Guiffre, S.; Axilbund, J.; Spiegel, A.; You, Y.N. The Increasing Incidence of Young-Onset Colorectal Cancer: A Call to Action. Mayo Clin. Proc. 2014, 89, 216–224. https://doi.org/10.1016/j.mayocp.2013.09.006.
- Al Zaabi, A.; Al Shehhi, A.; Sayed, S.; Al Adawi, H.; Al Faris, F.; Al Alyani, O.; Al Asmi, M.; Al-Mirza, A.; Panchatcharam, S.; Al-Shaibi, M. Early Onset Colorectal Cancer in Arabs, Are We Dealing with a Distinct Disease? Cancers 2023, 15, 889. https://doi.org/10.3390/cancers15030889.
- Cavestro, G.M.; Mannucci, A.; Zuppardo, R.A.; Di Leo, M.; Stoffel, E.; Tonon, G. Early onset sporadic colorectal cancer: Wor-risome trends and oncogenic features. Dig. Liver Dis. 2018, 50, 521–532. https://doi.org/10.1016/j.dld.2018.02.009.
- Hadjiliadis, D.; Khoruts, A.; Zauber, A.G.; Hempstead, S.E.; Maisonneuve, P.; Lowenfels, A.B.; Braid, A.L.; Cullina, J.; Dag-gett, A.; Fink, A.; et al. Cystic Fibrosis Colorectal Cancer Screening Consensus Recommendations. Gastroenterology 2018, 154, 736-745.e14. https://doi.org/10.1053/j.gastro.2017.12.012.
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeff-inger, K.C.; Shih, Y.-C.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society: ACS Colorectal Cancer Screening Guideline. CA A Cancer J. Clin. 2018, 68, 250–281. https://doi.org/10.3322/caac.21457.
- Carethers, J.M. Screening for Colorectal Cancer in African Americans: Determinants and Rationale for an Earlier Age to Commence Screening. Dig. Dis. Sci. 2015, 60, 711–721. https://doi.org/10.1007/s10620-014-3443-5.
- Gini, A.; Meester, R.G.S.; Keshavarz, H.; Oeffinger, K.C.; Ahmed, S.; Hodgson, D.C.; Lansdorp-Vogelaar, I. Cost-Effectiveness of Colonoscopy-Based Colorectal Cancer Screening in Childhood Cancer Survivors. JNCI J. Natl. Cancer Inst. 2019, 111, 1161–1169. https://doi.org/10.1093/jnci/djz060.
