Hyperlipidemia is a major risk factor for atherosclerotic diseases. Experimental animals play an important role in elucidating the molecular mechanisms of the pathophysiology of hyperlipidemia as well as in drug development. Rabbits are one of the most suitable models to study human hyperlipidemia because many features of the lipoprotein metabolism of rabbits are similar to those of humans. Currently, three types of rabbit models are commonly used for studying hyperlipidemia: (1) diet-induced hyperlipidemic rabbits, (2) spontaneous hyperlipidemic rabbits, and (3) gene-manipulated rabbits (transgenic and knockout rabbits).
- hyperlipidemia
- dyslipidemia
- atherosclerosis
- lipoprotein
- apolipoprotein
- rabbit
1. Introduction
Hyperlipidemia is one of the major risk factors for atherosclerosis, which has been the leading cause of mortality globally [1,2]. It is generally believed that an increase in plasma low-density lipoprotein (LDL), triglycerides (TG)-rich lipoproteins such as chylomicron (CM), and very-low-density lipoprotein (VLDL) and/or a decrease in high-density lipoprotein (HDL) are associated with or cause atherosclerosis [3]. For the study of human lipid disorders as well as for the development of therapeutic agents, it is essential to use an appropriate experimental animal. Ideal animal models for human hyperlipidemia should possess several important characteristics: (1) they should be easy to induce hyperlipidemia by diet intervention or genetic manipulation, (2) they should have similar lipoprotein profiles as humans, (3) they should be easy to handle and be of the proper size to allow for all anticipated experimental manipulations, and (4) they should be easy to acquire and maintain at a reasonable cost [4]. Until now, many animal models have been used for the study of hyperlipidemia, including mice, rats, hamsters, guinea pigs, rabbits, pigs, and nonhuman primates. Unfortunately, there is no single animal model that fulfills all the requirements. Although each animal model has its advantages and limitations with respect to plasma lipoprotein profiles, handling, reproducibility, and cost, rabbits possess several unique advantages for the study of lipid metabolism. Due to their high susceptibility to a cholesterol diet, it is easy to induce hyperlipidemia and atherosclerosis in wild-type rabbits [5], which is different from most strains of wild-type mice. The hyperlipidemic models of mice have been generated by the targeting of genes, such as apolipoprotein (apo) E and LDL receptor (LDLR) [6,7]. Nevertheless, there are a number of features that make rabbits an appropriate model to study human hyperlipidemia. Unlike wild-type mice and rats (rodents) in which HDL is a major lipoprotein in plasma, ≈40% of plasma cholesterol in wild-type rabbits and > 90% in cholesterol-fed rabbits are contained in the apo B-containing particles such as VLDL and LDL [8]. Humans and rabbits have abundant plasma cholesteryl ester transfer protein (CETP), an important regulator of HDL and cholesterol metabolism, whereas rodents do not have CETP [9]. Given that the restricted editing of apo B mRNA only occurs in the intestine in humans and rabbits, apo B-48 is only present in intestinally derived CM and CM remnants in humans and rabbits. However, in rodents, apo B mRNA editing occurs in both the intestine and liver [10]; therefore, apo B-48 is contained in both CM and VLDL particles. Furthermore, hepatic LDLR in both humans and rabbits is highly down-regulated according to the level of cholesterol uptake in the liver [8]. In addition, the appropriate size of rabbits enables researchers to obtain large amounts of plasma samples for both in vitro and in vivo studies.
2. Hyperlipidemic Rabbit Models
2.1. Diet-Induced Hyperlipidemic Rabbits
2.1.1. Cholesterol-Fed Rabbits
Rabbits are the first models for the study of lipoprotein metabolism and atherosclerosis. In 1913, a Russian experimental pathologist, Anitschow, described that feeding cholesterol dissolved in sunflower oil to rabbits elevated blood cholesterol levels, and within several weeks, rabbit arteries showed atherosclerotic lesions [11]. As herbivores, laboratory rabbits including New Zealand white (NZW) and Japanese white (JW) rabbits, on a normal standard diet, have relatively low plasma total cholesterol (TC) levels (30–90 mg/dL) at the age of 3–4 months compared with humans. When rabbits are fed a cholesterol diet, they rapidly develop hypercholesterolemia [12]. Kolodgie et al. tested low dietary cholesterol (0.05% to 0.25%) with 6% peanut oil for 30 weeks to explore a response of plasma TC levels in rabbits. Dietary cholesterol in the range of 0.05% to 0.15% resulted in a less than 2-fold stepwise increase in plasma TC, whereas rabbits receiving 0.20% and 0.25% dietary cholesterol showed significantly higher (4- to 5-fold) plasma TC compared to those rabbits fed a diet containing 0.05% to 0.15% cholesterol [13]. Cholesterol diets contain more than 1% cholesterol usually causes extraordinary elevation of plasma TC levels in rabbits, exceeding 2000 mg/dL. Such high level of plasma TC is never seen in human hypercholesterolemia. Therefore, it is generally recommended to use a 0.3–0.5% cholesterol diet, which results in hypercholesterolemia comparable to human familial hypercholesterolemia (FH) [8]. The major elevated lipoproteins in cholesterol-fed rabbits are those lipoproteins (called β-VLDL) derived from both the intestine and liver and they are quite atherogenic because they are rich in cholesteryl esters and can induce macrophages to transform into foam cells [14].
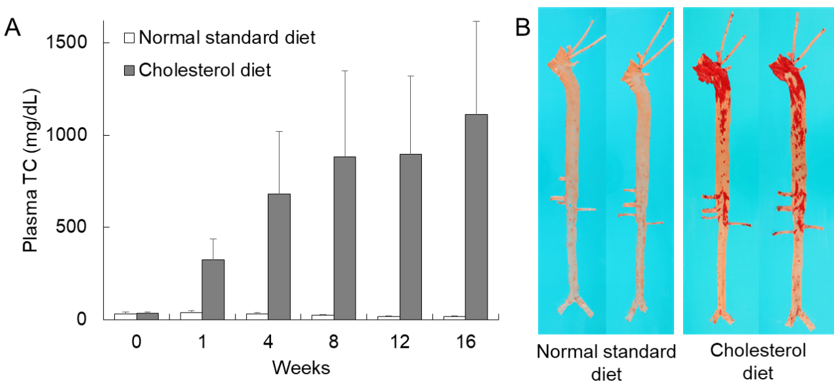
Currently, we recommend a diet supplemented with 0.3–0.5% cholesterol and 3% soybean oil fed either ad libitum or restricted for most rabbit experiments. Representative hypercholesterolemia in cholesterol-fed rabbits is shown in Figure 1A. Plasma TC levels start to rise within one week and remain at high levels (≈800 mg/dL) thereafter. Hyperlipidemic rabbits can develop aortic lesions as early as 4–6 weeks after cholesterol diet feeding, but at 16 weeks, 20–40% of the aortic surface is covered by atherosclerosis which can be stained with Sudan IV (Figure 1B). The age of rabbits should be considered because young rabbits are more susceptible to aortic atherosclerosis than old rabbits even though there are no differences in plasma TC levels [15].
Figure 1. Hypercholesterolemia and representative aortic atherosclerosis of cholesterol-fed rabbits. (A) Plasma total cholesterol (TC) levels of wild-type rabbits fed either a normal standard diet or a cholesterol diet containing 0.3% cholesterol and 3% soybean oil for 16 weeks. Mean ± SD (n = 4–20). (B) Aortic gross lesion stained with Sudan IV can be seen in rabbits fed a cholesterol diet (right).
2.1.2. Casein-Fed Rabbits
A cholesterol-free, casein-enriched diet can also induce hypercholesterolemia and atherosclerosis in rabbits. In general, hypercholesterolemia is induced in rabbits by feeding them a semi-purified diet enriched in 27% casein, and plasma TC levels are increased up to 300–800 mg/dL and accompanied by aortic atherosclerosis [16,17]. This model is seldom used; however, the possible mechanism for hypercholesterolemia is considered as being caused by decreased bile acids synthesis and fecal sterol excretion, which leads to increased hepatic cholesterol, followed by down-regulation of LDLR [18–20]. The major elevated lipoproteins in casein-fed rabbits are LDLs, which is different from β-VLDLs present in cholesterol-fed rabbits. Daley et al. compared casein-fed rabbits with cholesterol-fed rabbits and found that even though with similar high hypercholesterolemia (≈500 mg/dL), casein-fed rabbits developed significantly less aortic atherosclerosis than cholesterol-fed rabbits [17].
2.2. Spontaneous Hyperlipidemic Rabbits
2.2.1. Watanabe Heritable Hyperlipidemic (WHHL) Rabbits
The WHHL rabbit was established by Dr. Watanabe in the 1970s at Kobe University in Japan [21,22] and is often used as a model of human familial hypercholesterolemia (FH). FH is an autosomal dominant genetic disorder characterized by elevated plasma LDL levels due to LDLR dysfunctions [23]. WHHL rabbits are genetically deficient in LDLR functions, therefore, even on a normal standard diet, they showed hyperlipidemia (plasma TC, 385–518 mg/dL, and TG, 304–511 mg/dL), being 10-fold and 8-fold higher than normal wild-type rabbits [22]. Serum lipoproteins characterized by electrophoresis exhibited elevated β-lipoprotein with a broad β-pattern and diminished α-lipoprotein in WHHL rabbits. Yamamoto et al. demonstrated that WHHL rabbits have a dysfunctional LDLR with an in-frame deletion of 12 nucleotides that eliminate four amino acids from the ligand-binding domain of the LDLR. Mutant LDLRs cannot transport to the cell surface at a normal rate [24]. The dysfunction of the LDLR in WHHL rabbits results in a loss of the hepatic uptake of LDL and subsequent elevation of the plasma LDL levels similar to human FH [25–27]. Age-related changes in plasma lipids were observed in WHHL rabbits [22,28]. Compared with 3-month-old juvenile rabbits, 24-month-old rabbits showed a 45% decrease in TC (916 at 3 months to 508 mg/dL at 24 months) and a 42% decrease in LDL-C (680 at 3 months to 393 mg/dL at 24 months) [28]. Atkinson et al. compared plasma TC levels in heterozygous WHHL rabbits and NZW rabbits on a cholesterol diet for 24 weeks. On a 0.5% cholesterol diet, plasma TC levels in heterozygous WHHL rabbits were significantly higher than those in NZW rabbits (≈2000 in WHHL vs. ≈1000 mg/dL in NZW rabbits). However, on a 1% cholesterol diet, plasma TC levels reached a peak (≈3000 mg/dL) at eight weeks in both rabbits without significant differences between the two groups [29]. Some WHHL rabbits (also designated as myocardial infarction-prone Watanabe heritable hyperlipidemic, WHHLMI) developed coronary atherosclerosis and myocardial infarction [8,30,31]. To obtain a myocardial infarction-prone colony, WHHL rabbits with severe coronary atherosclerosis, myocardial infarction, and relatively higher plasma TC levels were selected and bred. Selective breeding was carried out for five to seven generations. WHHLMI rabbits exhibit 93% of coronary stenosis and 97% of myocardial infarction, whereas the corresponding values are 60% and 23% in original WHHL rabbits [30].
2.2.2. St. Thomas’ Mixed Hyperlipidemic (SMHL) Rabbits
The SMHL rabbit is a putative model of familial combined hyperlipidemia originally described as the St. Thomas’ Hospital rabbit in the 1980s. These rabbits showed spontaneously elevated plasma TC levels (394 mg/dL, 4- to 5-fold over normal rabbits) with moderately high or normal plasma TG levels (151 mg/dL, 2-fold over normal rabbits) and developed aortic atherosclerosis on a normal standard diet [32,33]. SMHL rabbits have normal LDLR function, and it is considered that elevated plasma VLDL and LDL levels are caused by overproduction of apoB-containing particles from the liver [32,34]. De Roos et al. compared plasma lipids of SMHL rabbits with WHHL rabbits on a low-cholesterol diet. With three months of feeding a 0.08% cholesterol diet, SMHL rabbits showed plasma TC of 264 ± 68 and TG of 290 ± 55 mg/dL compared with the TC of 791 ± 36 and TG of 232 ± 46 mg/dL in WHHL rabbits [35].
2.2.3. Postprandial Hypertriglyceridemic (PHT) Rabbits
The PHT rabbit was established through cross-breeding between WHHL rabbits with a hypertriglyceridemia phenotype and normal JW rabbits [36,37]. PHT rabbits showed high TG levels in both fasting (403 mg/dL, 11-fold over normal rabbits) and postprandial (1407 mg/dL, 22-fold over normal rabbits) states. In addition to hypertriglyceridemia, PHT rabbits exhibited insulin resistance along with obesity [37].
2.3. Gene-Manipulated Rabbits
2.3.1. Transgenic (Tg) Rabbits
Hyperlipidemic rabbits were also generated by the overexpression of human apo B-100, E, and C-III genes in the liver. Human apo B-100 Tg rabbits resulted in a 3-fold increase in plasma TC and TG levels compared with those in non-Tg rabbits, and the majority of the plasma TC was distributed in the LDL, with striking enrichment of TG content [38]. Ding et al. generated Tg rabbits overexpressing the human apo C-III gene. Apo C-III Tg rabbits showed 3-fold higher plasma TG levels than non-Tg rabbits (191 in Tg vs. 59 mg/dL in non-Tg), although TC and HDL-C levels were not changed. Lipoprotein analysis revealed that increased TG in apo C-III Tg rabbits was distributed in CM and VLDL [39]. Tg rabbits expressing human apo E3, the most common isoform in humans, and apo E2, a variant associated with type III hyperlipoproteinemia, were also reported. Tg rabbits expressing higher levels of human apo E3 (>20 mg/dL) showed marked combined hyperlipidemia characterized by an increase in both LDL and VLDL [40,41]. Overexpression of human apo E2 in rabbits exhibited 8-fold increases in plasma TC and 15-fold increases in plasma TG compared with non-Tg rabbits [42].
2.3.2. Knockout (KO) Rabbits
By using gene-editing technologies, apo E and LDLR KO rabbits were generated and have been used for the study of hyperlipidemia [43–45]. ApoE-KO rabbits exhibited mild hyperlipidemia, with TC levels at ≈200 mg/dL, on a normal standard diet [44,46]. When challenged with a cholesterol diet, apo E KO rabbits showed greater susceptibility to hyperlipidemia than did the wild-type rabbits, and their plasma TC and TG levels were remarkably increased, with a 6.3-fold increase in TC and a 5.7-fold increase in TG compared with those of wild-type rabbits [46]. Hyperlipidemia in apo E KO rabbits was caused by elevated remnant lipoproteins predominated by apo B-48 and rich in both apo A-I and apo A-IV contents. Lu et al. generated LDLR KO rabbits and found that, similar to WHHL rabbits, LDLR deficiency markedly increased plasma TC levels (272–1013 in LDLR-KO rabbits vs. 51 mg/dL in the wild-type rabbits) on a normal standard diet. Increased plasma TC levels were mainly caused by elevated LDL-C (124–730 in LDLR-KO rabbits vs. 21 mg/dL in the wild-type rabbits) [45]. Apo E and LDLR double-KO rabbits were also generated by the same group and exhibited remarkable hyperlipidemia on a normal standard diet [47].
References
- Ross, R. Atherosclerosis — An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126, doi:10.1056/NEJM199901143400207.
- Hansson, G.K. Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 2005, 352, 1685–1695, doi:10.1056/NEJMra043430.
- Beaumont, J.L.; Carlson, L.A.; Cooper, G.R.; Fejfar, Z.; Fredrickson, D.S.; Strasser, T. Classification of hyperlipidaemias and hyperlipoproteinaemias. Bull. World Health Organ. 1970, 43, 891–915, doi:10.1161/01.cir.45.2.501.
- Fan, J.; Chen, Y.; Yan, H.; Niimi, M.; Wang, Y.; Liang, J. Principles and applications of rabbit models for atherosclerosis research. J. Atheroscler. Thromb. 2018, 25, 213–220.
- Duff, G.L. Experimental cholesterol arteriosclerosis and its relationship to human arteriosclerosis. Arch Pathol 1935, 20, 81–123.
- Zhang, S.H.; Reddick, R.L.; Piedrahita, J.A.; Maeda, N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 1992, 258, 468–471, doi:10.1126/science.1411543.
- Ishibashi, S.; Brown, M.S.; Goldstein, J.L.; Gerard, R.D.; Hammer, R.E.; Herz, J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest. 1993, 92, 883–893, doi:10.1172/JCI116663.
- Fan, J.; Kitajima, S.; Watanabe, T.; Xu, J.; Zhang, J.; Liu, E.; Chen, Y.E. Rabbit models for the study of human atherosclerosis: from pathophysiological mechanisms to translational medicine. Pharmacol. Ther. 2015, 146, 104–19, doi:10.1016/j.pharmthera.2014.09.009.
- Ha, Y.; Barter, P. Differences in plasma cholesteryl ester transfer activity in sixteen vertebrate species. Comp. Biochem. Physiol. -- Part B Biochem. 1982, 71, 265–269, doi:10.1016/0305-0491(82)90252-8.
- Greeve, J.; Altkemper, I.; Dieterich, J.H.; Greten, H.; Windler, E. Apolipoprotein B mRNA editing in 12 different mammalian species: Hepatic expression is reflected in low concentrations of apoB-containing plasma lipoproteins. J. Lipid Res. 1993, 34, 1367–1383.
- Steinberg, D. Thematic review series: Living history of lipids: In celebration of the 100th anniversary of the lipid hypothesis of atherosclerosis. J. Lipid Res. 2013, 54, 2946–2949.
- Fan, J.; Watanabe, T. Cholesterol-fed and transgenic rabbit models for the study of atherosclerosis. J. Atheroscler. Thromb. 2000, 7, 26–32, doi:10.5551/jat1994.7.26.
- Kolodgie, F.D.; Katocs, A.S.; Largis, E.E.; Wrenn, S.M.; Cornhill, J.F.; Herderick, E.E.; Lee, S.J.; Virmani, R. Hypercholesterolemia in the Rabbit Induced by Feeding Graded Amounts of Low-Level Cholesterol. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 1454–1464, doi:10.1161/01.ATV.16.12.1454.
- Mahley, R.W.; Innerarity, T.L.; Brown, M.S.; Ho, Y.K.; Goldstein, J.L. Cholesteryl ester synthesis in macrophages: stimulation by beta-very low density lipoproteins from cholesterol-fed animals of several species. J. Lipid Res. 1980, 21, 970–980.
- Keyamura, Y.; Nagano, C.; Kohashi, M.; Niimi, M.; Nozako, M.; Koyama, T.; Itabe, H.; Yoshikawa, T. Dietary cholesterol atherogenic changes in juvenile rabbits. Biol. Pharm. Bull. 2015, 38, 785–788, doi:10.1248/bpb.b14-00775.
- Huff, M.W.; Hamilton, R.M.G.; Carroll, K.K. Plasma cholesterol levels in rabbits fed low fat, cholesterol-free, semipurified diets: effects of dietary proteins, protein hydrolysates and amino acid mixtures. Atherosclerosis 1977, 28, 187–195, doi:10.1016/0021-9150(77)90156-3.
- Daley, S.J.; Herderick, E.E.; Cornhill, J.F.; Rogers, K.A. Cholesterol-fed and casein-fed rabbit models of atherosclerosis - Part 1: Differing lesion area and volume despite equal plasma cholesterol levels. Arterioscler. Thromb. 1994, 14, 95–104, doi:10.1161/01.ATV.14.1.95.
- Beynen, A.C.; Van der Meer, R.; West, C.E. Mechanism of casein-induced hypercholesterolemia: Primary and secondary features. Atherosclerosis 1986, 60, 291–293.
- Huff, M.W.; Carroll, K.K. Effects of dietary protein on turnover, oxidation, and absorption of cholesterol, and on steroid excretion in rabbits. J. Lipid Res. 1980, 21, 546–48.
- Chao, Y.; Yamin, T.T.; Alberts, A.W. Effects of cholestyramine on low density lipoprotein binding sites on liver membranes from rabbits with endogenous hypercholesterolemia induced by a wheat starch-casein diet. J. Biol. Chem. 1982, 257, 3623–7.
- Watanabe, Y.; Ito, T.; Kondo, T. Breeding of a Rabbit Strain of Hyperlipidemia and Characteristic of These Strain. Exp. Anim. 1977, 26, 35–41, doi:10.1538/expanim1957.26.1_35.
- Watanabe, Y. Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit). Incidence and development of atherosclerosis and xanthoma. Atherosclerosis 1980, 36, 261–268, doi:10.1016/0021-9150(80)90234-8.
- Soutar, A.K.; Naoumova, R.P. Mechanisms of disease: Genetic causes of familial hypercholesterolemia. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 214–225, doi:10.1038/ncpcardio0836.
- Yamamoto, T.; Bishop, R.W.; Brown, M.S.; Goldstein, J.L.; Russell, D.W. Deletion in Cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science 1986, 232, 1230–1237, doi:10.1126/science.3010466.
- Kita, T.; Brown, M.S.; Watanabe, Y.; Goldstein, J.L. Deficiency of low density lipoprotein receptors in liver and adrenal gland of the WHHL rabbit, an animal model of familial hypercholesterolemia. Proc. Natl. Acad. Sci. U. S. A. 1981, 78, 2268–2272, doi:10.1073/pnas.78.4.2268.
- Havel, R.J.; Kita, T.; Kotite, L.; Kane, J.P.; Hamilton, R.L.; Goldstein, J.L.; Brown, M.S. Concentration and composition of lipoproteins in blood plasma of the WHHL rabbit. An animal model of human familial hypercholesterolemia. Arteriosclerosis 1982, 2, 467–474, doi:10.1161/01.atv.2.6.467.
- Goldstein, J.L.; Kita, T.; Brown, M.S. Defective Lipoprotein Receptors and Atherosclerosis: Lessons from an Animal Counterpart of Familial Hypercholesterolemia. N. Engl. J. Med. 1983, 309, 288–296, doi:10.1056/NEJM198308043090507.
- Shiomi, M.; Ito, T.; Fujioka, T.; Tsujita, Y. Age-associated decrease in plasma cholesterol and changes in cholesterol metabolism in homozygous Watanabe heritable hyperlipidemic rabbits. Metabolism 2000, 49, 552–556, doi:10.1016/S0026-0495(00)80025-6.
- Atkinson, J.B.; Hoover, R.L.; Berry, K.K.; Swift, L.L. Cholesterol-fed heterozygous Watanabe heritable hyperlipidemic rabbits: a new model for atherosclerosis. Atherosclerosis 1989, 78, 123–136, doi:10.1016/0021-9150(89)90216-5.
- Shiomi, M.; Ito, T.; Yamada, S.; Kawashima, S.; Fan, J. Development of an Animal Model for Spontaneous Myocardial Infarction (WHHLMI Rabbit). Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1239–1244, doi:10.1161/01.ATV.0000075947.28567.50.
- Shiomi, M.; Fan, J. Unstable coronary plaques and cardiac events in myocardial infarction-prone Watanabe heritable hyperlipidemic rabbits: questions and quandaries. Curr. Opin. Lipidol. 2008, 19, 631–636, doi:10.1097/MOL.0b013e3283189c18.
- La Ville, A.; Turner, P.R.; Pittilo, R.M.; Martini, S.; Marenah, C.B.; Rowles, P.M.; Morris, G.; Thomson, G.A.; Woolf, N.; Lewis, B. Hereditary hyperlipidemia in the rabbit due to overproduction of lipoproteins. I. Biochemical studies. Arteriosclerosis 1987, 7, 105–112, doi:10.1161/01.atv.7.2.105.
- Seddon, A.M.; Woolf, N.; La Ville, A.; Pittilo, R.M.; Rowles, P.M.; Turner, P.R.; Lewis, B. Hereditary hyperlipidemia and atherosclerosis in the rabbit due to overproduction of lipoproteins. II. Preliminary report of arterial pathology. Arteriosclerosis 1987, 7, 113–124, doi:10.1161/01.atv.7.2.113.
- Ardern, H.A.; Benson, G.M.; Suckling, K.E.; Caslake, M.J.; Shepherd, J.; Packard, C.J. Apolipoprotein B overproduction by the perfused liver of the St. Thomas’ mixed hyperlipidemic (SMHL) rabbit. J. Lipid Res. 1999, 40, 2234–2243.
- de Roos, B.; Caslake, M.J.; Milliner, K.; Benson, G.M.; Suckling, K.E.; Packard, C.J. Characterisation of the lipoprotein structure in the St. Thomas’ Mixed Hyperlipidaemic (SMHL) rabbit. Atherosclerosis 2005, 181, 63–68, doi:10.1016/j.atherosclerosis.2005.01.008.
- Mitsuguchi, Y.; Ito, T.; Ohwada, K. Pathologic findings in rabbit models of hereditary hypertriglyceridemia and hereditary postprandial hypertriglyceridemia. Comp. Med. 2008, 58, 465–480.
- Kawai, T.; Ito, T.; Ohwada, K.; Mera, Y.; Matsushita, M.; Tomoike, H. Hereditary postprandial hypertriglyceridemic rabbit exhibits insulin resistance and central obesity: A novel model of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2752–2757, doi:10.1161/01.ATV.0000245808.12493.40.
- Fan, J.; McCormick, S.P.A.; Krauss, R.M.; Taylor, S.; Quan, R.; Taylor, J.M.; Young, S.G. Overexpression of Human Apolipoprotein B-100 in Transgenic Rabbits Results in Increased Levels of LDL and Decreased Levels of HDL. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1889–1899, doi:10.1161/01.ATV.15.11.1889.
- Ding, Y.; Wang, Y.; Zhu, H.; Fan, J.; Yu, L.; Liu, G.; Liu, E. Hypertriglyceridemia and delayed clearance of fat load in transgenic rabbits expressing human apolipoprotein CIII. Transgenic Res. 2011, 20, 867–875, doi:10.1007/s11248-010-9467-5.
- Fan, J.; Ji, Z.S.; Huang, Y.; de Silva, H.; Sanan, D.; Mahley, R.W.; Innerarity, T.L.; Taylor, J.M. Increased expression of apolipoprotein E in transgenic rabbits results in reduced levels of very low density lipoproteins and an accumulation of low density lipoproteins in plasma. J. Clin. Invest. 1998, 101, 2151–2164, doi:10.1172/JCI1599.
- Huang, Y.; Ji, Z.-S.; Brecht, W.J.; Rall, S.C.; Taylor, J.M.; Mahley, R.W. Overexpression of Apolipoprotein E3 in Transgenic Rabbits Causes Combined Hyperlipidemia by Stimulating Hepatic VLDL Production and Impairing VLDL Lipolysis. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2952–2959, doi:10.1161/01.ATV.19.12.2952.
- Huang, Y.; Schwendner, S.W.; Rall, S.C.; Sanan, D.A.; Mahley, R.W. Apolipoprotein E2 Transgenic Rabbits. J. Biol. Chem. 1997, 272, 22685–22694, doi:10.1074/JBC.272.36.22685.
- Yang, D.; Xu, J.; Zhu, T.; Fan, J.; Lai, L.; Zhang, J.; Chen, Y.E. Effective gene targeting in rabbits using RNA-guided Cas9 nucleases. J. Mol. Cell Biol. 2014, 6, 97–99, doi:10.1093/jmcb/mjt047.
- Ji, D.; Zhao, G.; Songstad, A.; Cui, X.; Weinstein, E.J. Efficient creation of an APOE knockout rabbit. Transgenic Res. 2015, 24, 227–235, doi:10.1007/s11248-014-9834-8.
- Lu, R.; Yuan, T.; Wang, Y.; Zhang, T.; Yuan, Y.; Wu, D.; Zhou, M.; He, Z.; Lu, Y.; Chen, Y.; et al. Spontaneous severe hypercholesterolemia and atherosclerosis lesions in rabbits with deficiency of low-density lipoprotein receptor (LDLR) on exon 7. EBioMedicine 2018, 36, 29–38, doi:10.1016/J.EBIOM.2018.09.020.
- Niimi, M.; Yang, D.; Kitajima, S.; Ning, B.; Wang, C.; Li, S.; Liu, E.; Zhang, J.; Chen, Y.E.; Fan, J. ApoE knockout rabbits: A novel model for the study of human hyperlipidemia. Atherosclerosis 2016, 245, 187–193, doi:10.1016/j.atherosclerosis.2015.12.002.
- Yuan, T.; Zhong, Y.; Wang, Y.; Zhang, T.; Lu, R.; Zhou, M.; Lu, Y.; Yan, K.; Chen, Y.; Hu, Z.; et al. Generation of hyperlipidemic rabbit models using multiple sgRNAs targeted CRISPR/Cas9 gene editing system. Lipids Health Dis. 2019, 18, 69, doi:10.1186/s12944-019-1013-8.
This entry is adapted from the peer-reviewed paper 10.3390/app10238681