Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Infective endocarditis (IE) is a growing epidemiological challenge. Appropriate diagnosis remains difficult due to heterogenous etiopathogenesis and clinical presentation. The disease may be followed by increased mortality and numerous diverse complications.
- infective endocarditis
- 18F-FDG PET/CT
- scintigraphy
1. Introduction
Infective endocarditis (IE) is considered to be a challenging disease both for initial evaluation and subsequent treatment due to complex etiopathogenesis and the presentation. Rising rates of cardiac implantable electronic devices (CIED) implantation in the elderly with the rising number of co-morbidities has led to an increase in the prevalence of cardiac device-related IE (CDRIE) cases [1,2,3,4,5]. Moreover, despite efforts towards accurate diagnosis and management, profound advances in microbiological testing, and multimodality imaging, the mortality rates stemming from IE have not decreased in more than two decades [6]. The infection extends to the electrode leads, native and prosthetic valves, and endocardium, forming vegetations composed of fibrin, microorganisms, platelets, and inflammatory cells [2]. As a result of the various causative microorganisms, intracardiac lesions, and coexisting comorbidities, IE may have an uncharacteristic clinical presentation, which hinders proper and timely diagnosis [7]. Signs and symptoms are including cardiac lesions, extracardiac embolic foci, immune-mediated reactions, and heart failure. Currently, there is no single reliable examination that can be conducted to establish a diagnosis and the evaluation includes diagnostic criteria depending on the type of the disease [8]. Since in selected populations, especially with CDRIE, Duke criteria have low diagnostic accuracy, due to high rates of negative microbiological testing results and difficulties in interpreting echocardiographic images with artifacts related to the prosthetic valves and electrodes, there have been in recent years efforts to establish novel IE and CDRIE criteria [2]. Indeed, inappropriate diagnosis may cause detrimental outcomes—inappropriate invasive procedures or delays in treatment [9].
2. 99mTc-HMPAO-SPECT/CT
The hybrid technique 99mTc-HMPAO-SPECT/CT relies upon the intracellular labeling of autologous white blood cells [16]. The HMPAO tracer and 99mTc radioisotope form a lipid-soluble complex, which passes through the cell membrane due to passive diffusion. Radiotracer remains in the cell as a result of the conversion of the complex into a hydrophilic one, by reducing agents such as glutathione, and binding to non-diffusible proteins and cell organelles [17]. White blood cell labeling has been made more accessible after introducing disposable sterile closed devices for the procedure. However, the process still remains time-consuming and necessitates blood handling [18]. Once administered intravenously radiolabeled white blood cells migrate to the respiratory system and, if not damaged, afterward to the liver, the spleen, and the reticuloendothelial tissues [16,17]. Following that, the migration is directed by chemotactic attraction to the bone marrow and infected sites [16,17]. Thus, 99mTc-HMPAO-SPECT/CT, is performed according to a 24-h-long protocol, including early (30–60 min), delayed (2–4 h), and late (20–24 h) acquisitions [16]. The accumulation may be influenced by antibiotic therapy, the type of pathogen, and the vascularization of the infected tissue [17]. Nevertheless, this technique provides high specificity, especially in the context of differentiating sterile and infectious morphological intracardiac lesions [19].
Autologous white blood cells can be radiolabeled ex-vivo using 99mTc-HMPAO or 111In-oxine. There is stronger evidence and wider application of 99mTc-HMPAO compared to 111In-oxine in this particular indication. Firstly, scintigraphy performed with 111In has poorer image quality and significantly higher radiation dose due to the long half-life time (6 h vs. 2.8 days) [16]. Moreover, a white blood cell scan performed with 99mTc-HMPAO has higher sensitivity and specificity compared to 111In-oxine (60–85% and 78–94%, for 111In-oxine; 96 and 92% for 99mTc-HMPAO) which has been noted in European Association of Nuclear Medicine procedural guidelines [16].
The 99mTc-HMPAO-SPECT/CT examination is deemed as positive for IE in the presence of at least one intracardiac and/or in the vicinity of the CIED electrode site of increased radiotracer uptake, and which is characterized by a time-dependent radioactivity pattern. Due to the specific characteristics of leucocyte biodistribution over time, it is possible to differentiate foci of increased tracer uptake which are diagnostic for infection [16,17]. White blood cells are observed in various organs and regions of bone marrow at specific points in time after their intravenous administration; however, the intensity of physiological uptake does not increase over time—in the delayed and late 99mTc-HMPAO-SPECT/CT acquisitions. In contrast, leucocytes display signs of accumulation in the infectious sites over a period of time. Therefore, it is crucial to evaluate lesions within a cardiovascular system suspected of IE in a time-dependent manner, including early, delayed, and late images.
Recent years have yielded more data justifying the application of this modality in IE evaluation, it was included for the first time in the recent ESC Guidelines within the diagnostic algorithm for PVE and in EHRA consensus in selected clinical scenarios-suspected CIED infection and coexisting positive blood cultures and negative echocardiography, for assessment of embolic events and in course of persistent sepsis after the procedure of device extraction (Figure 1 and Figure 2) [2,15]. In clinical practice distinction between CDRIE, including cardiac tissues and/or the intravascular portion of the lead and local device infection (LDI), limited solely to the CIED lodge, is crucial in terms of differences in regard to prognosis and treatment. Patients with CDRIE have an increase of 15–20% in mortality after 1 year and require a prolonged course of intravenous antibiotic therapy combined with complete hardware removal [2,4,20,21].
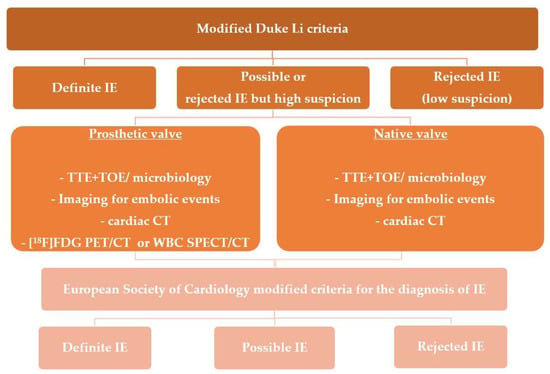
Figure 1. Shows indications for molecular imaging techniques in patients with suspected infective endocarditis.
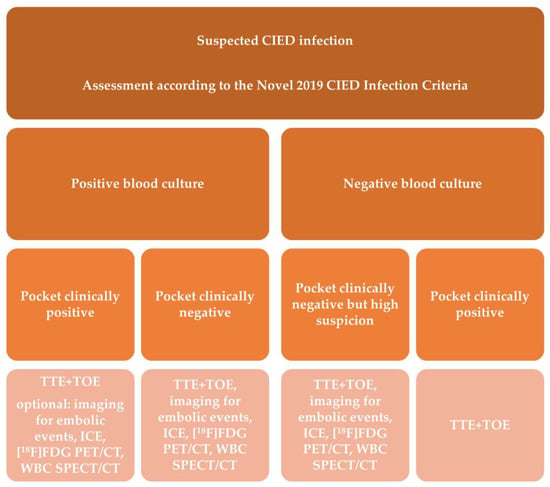
Figure 2. Shows indications for molecular imaging techniques in patients with suspected cardiac device-related infective endocarditis.
The 99mTc-HMPAO-SPECT/CT examination has high specificity and sensitivity in suspected PVE, especially in case of inconclusive transthoracic echocardiography (TTE) [22,23,24]. The diagnostic accuracy of this technique was validated based on histopathological examination as a reference gold standard [25]. The 99mTc-HMPAO-SPECT/CT supports the more accurate reclassification of patients with suspected PVE [25]. Moreover, it has excellent diagnostic value in the visualization of perivalvular abscesses [25]. As a result, 99mTc-HMPAO-SPECT/CT was included in IE diagnostic criteria and algorithm in ESC Guidelines in patients with suspected PVE [2]. The diagnostic parameters in solely the NVE group have not been validated so far [2].
The diagnostic properties of 99mTc-HMPAO-SPECT/CT in cardiac device-related infections were evaluated in multiple studies [26,27,28,29,30]. It is characterized cumulatively by 60–93.7% sensitivity, 88–100% specificity, 84.6–93.9% negative predictive value (NPV), and 74–100% positive predictive value (PPV) [31]. Besides the extent of device involvement, the detected extracardiac inflammatory lesions were diagnosed as: extracardiac concomitant infection, initial sites of infection leading to CDRIE, or coexisting inflammatory lesions [31]. The 99mTc-HMPAO-SPECT/CT examination is able to reliably exclude CDRIE in patients with fever or sepsis (95% NPV) [27]. The inclusion of the 99mTc-HMPAO-SPECT/CT positive result as a major criterion substantially improves the initial Duke-Li classification [24,26]. Moreover, in CDRIE suspicion, positive 99mTc-HMPAO-SPECT/CT results are associated with increased rates of in-hospital mortality (11.4% vs. 0%, respectively; odds ratio: 19.6; 95% confidence interval [CI]: 1.02 to 374.70), complications (43% vs. 9%, respectively; hazard ratio [HR]: 5.9; 95% CI: 2.27 to 15.20), and procedure of a hardware removal (57% vs. 16%, respectively; HR: 4.3; 95% CI: 2.07 to 19.08) [30].
In a prospective study assessing whether prior antimicrobial treatment impacts the diagnostic profile of this modality in IE diagnosis, this technique displayed 81.95% accuracy, 86.92% specificity, 73.33% sensitivity, 84.96% NPV, and 76.39% PPV [32]. Importantly, antimicrobial therapy was related to higher rates of false-negative 99mTc-HMPAO-SPECT/CT results (OR, 4.63; 95% CI, 1.41 to 15.23, p = 0.01). Given that prolonged pharmacotherapy is one of the crucial elements in managing patients with IE, it is vital to understand its impact on the diagnostic tools (Figure 3 and Figure 4).
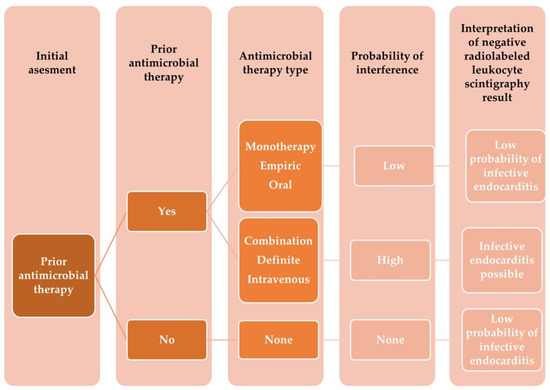
Figure 3. Shows the proposed algorithm on how to interpret a negative 99mTc-HMPAO-SPECT/CT results in patients with suspected infective endocarditis receiving antimicrobial therapy prior to examination.
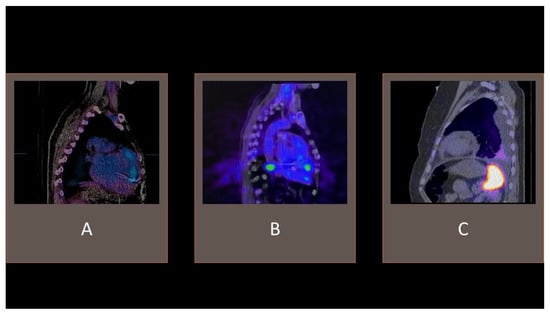
Figure 4. Examples of 99mTc-HMPAO-SPECT/CT. (A) tracer uptake consistent with cardiac device-related infective endocarditis with an accumulation of radiolabeled leucocytes in the vicinity of an implanted electrode. (B) visible tracer uptake in the vicinity of an implanted left ventricle assist device. (C) accumulation of radiolabeled leucocytes in the pleural cavity effusion, without pathological intracardiac uptake.
3. 18F-FDG PET/CT
The 18F-FDG PET/CT, relies on the radiotracer accumulating in cells with high numbers of metabolically active glucose transporters expressed on their cell surface—activated inflammatory cells such as macrophages and lymphocytes. Administered 18F-FDG passes to the cell via glucose transporters (GLUTs), subsequently is phosphorylated by hexokinases (HXKs) to FDG-6-phosphate, afterward remaining in the cell [33]. The recommended administered activity is at a level of 2.5–5 MBq/kg, which corresponds with 175 to 350MBq for an adult weighing 70 kg [34]. The acquisition should be performed 60 min after the radiotracer administration [35]. The 18F-FDG PET/CT images should be evaluated in 2D planes and in 3D maximum intensity projection cine mode, in terms of intensity and the pattern of the uptake. The heterogeneous uptake can be associated with an infection [36]. Moreover, 18F-FDG PET/CT provides quantification opportunities and extracardiac septic foci assessment [37,38].
The diagnostic accuracy of this technique is depending on the proper suppression of the natural radiotracer myocardial uptake by means of a low-carbohydrate and high-fat diet, followed by a period of fast [35]. Although the acquisition itself is short, this technique requires time-consuming patient preparation. The Society of Nuclear Medicine and Molecular Imaging (SNMMI)/American Society of Nuclear Cardiology (ASNC)/Society of Cardiovascular CT (SCCT) guidelines advise a fat-enriched diet without carbohydrates for 12–24 h prior to the examination, a fast of 12–18 h, followed by the administration of intravenous heparin 15 min prior to radiotracer administration [39]. Nonetheless, it should be noted that in many published studies there are applied variable times of dietary restrictions and pharmacological procedures for study preparation [31]. Those various myocardial suppression protocols ought to be acknowledged in the context of discrepancies in 18F-FDG PET/CT diagnostic accuracy in IE work-up. Unification of imaging protocols and standardization of procedures is of paramount importance for providing reliable data for continuous evaluation and further development of those techniques. Moreover, numerous lesions may imitate IE-like uptake—primary and metastatic cardiac tumors, vasculitis, inflammation related to surgical procedures, and foreign body reactions—typically in patients with PVE, as a result of the use of a local tissue adhesive [35].
Currently 18F-FDG PET/CT results are included in PVE diagnostic criteria and algorithm, as well as in the Novel 2019 International CIED Infection Criteria [2,15]. This modality is recommended in patients with suspected CDRIE, positive blood cultures, and negative echocardiography, as well as for identification of extracardiac foci [15]. Additionally, it should be performed in the case of S. aureus bacteremia in patients with CIED, and for identification of the infection portal of entry [15].
Based on meta-analysis results 18F-FDG PET/CT has rather limited pooled sensitivity for the NVE diagnosis, while pooled specificity is high—36% and 99%, respectively [40]. The low spatial resolution of PET/CT imaging reaching 5 mm is considered to be an important limitation for the detection of small vegetations with continuous cardiac movements [41]. Nonetheless, the diagnostic performance of this modality in PVE evaluation was investigated in a meta-analysis including 15 studies with 333 cases, confirming high pooled sensitivity and specificity were at levels of 86% and 84%, respectively [42]. The 18F-FDG PET/CT supports the more accurate reclassification of patients with suspected PVE [43,44]. Moreover, a simultaneous acquisition with electrocardiogram-gated CT angiography (CTA) should be considered for increasing anatomical resolution [45]. However, a recent study including patients with all types of disease evaluating a 4-point scoring system showed the sensitivity, specificity, PPV, NPV, and accuracy of the qualitative approach were 93%, 81%, 84%, 91%, and 87%, respectively [46].
There are numerous studies evaluating the diagnostic properties of 18F-FDG PET/CT in CDRIE diagnosis [47,48,49,50,51,52,53,54,55,56,57,58,59]. This modality is characterized cumulatively by 86.67–93% accuracy, 62.5–100% specificity, 30.8–100% sensitivity, 66–100% PPV, and 75–100% NPV for CDRIE diagnosis [31]. However, 18F-FDG PET/CT presents 86.6% accuracy, 72.2–84.2% sensitivity, 95.6–100% specificity, 86.7–94.1% PPV, 88.9–89.6% NPV while detecting LDI [31]. Importantly, false-negative imaging results occurred in patients who had undergone previous antimicrobial therapy [51,54,55]. Assessment of the maximum standardized uptake values (SUVmax) may provide additional insight into ongoing infections [47,49,50,51,52,53,54,55,56,59]. Results support mainly SUVmax assessment for device pocket infection diagnosis. The value of SUVmax of the lodge was significantly higher in the case of CIED-related infection compared to the control group (4.8 ± 2.4 vs. 2.0 ± 0.8, p < 0.001) [47]. Moreover, in patients with microbiologically confirmed lodge involvement, there was a substantial increase of mean local SUVmax of delayed acquisition compared to standard one (4.73 ± 1.72 vs. 3.51 ± 1.92, p = 0.0002), followed by a mean lead SUVmax significant increase compared to standard acquisition in patients with infected leads (3.25 ± 0.93 vs. 1.11 ± 1.70, p = 0.01) [52]. Moreover, the identification of patients with a “Cold Closed Pocket” (i.e., a negative CIED pocket in 18F-FDG PET/CT without skin erosion/perforation) may be clinically relevant, since this subset of patients presents worse long-term survival [55].
This entry is adapted from the peer-reviewed paper 10.3390/vaccines11020420
This entry is offline, you can click here to edit this entry!
