Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
In bioleaching, the function of the solvent is performed by microorganisms, by the action of either bacteria or fungi, as they participate in the biogeochemical cycle of minerals in direct ways by the metabolism of the microorganisms or indirectly by the products of their metabolism. Therefore, bioleaching is defined as the solubilization of metals from insoluble solid substrates.
- biological extraction
- metals
- bioprocess
- bioleaching
- bacterial bioleaching
- fungal bioleaching
1. Bioleaching
Leaching is a chemical method for extracting metals of interest [1]. Leaching, peroration, or solid/liquid extraction all involve an operation that consists of extracting with the help of a potentially soluble component a solute that is in solid form accompanied by other undesirable solids [2].
In general, in a leaching process, there are three components:
- A: solid solute that goes into the solution;
- B: inert solid (insoluble in S);
- S: extracting solvent.
In bioleaching, the function of the solvent is performed by microorganisms, by the action of either bacteria or fungi, as they participate in the biogeochemical cycle of minerals in direct ways by the metabolism of the microorganisms or indirectly by the products of their metabolism [3][4].
Therefore, bioleaching is defined as the solubilization of metals from insoluble solid substrates [3]. In the case of employing bacteria, they use the mineral as a substrate, capturing electrons for their metabolic processes and releasing heat and metals without needing any external energy to carry out the process [5]. In the case of fungi, they produce organic acids, which leach metals from solid matrices. Microorganisms can also excrete ligands that stabilize the metal by forming metal-rich complexes [6].
Metal solubilization can be facilitated by biologically produced amino acids, cyanide, and thiosulfate [6]. In addition, microorganisms can participate in the redox cycling of iodine [7], which is a potential alternative leachant for obtaining the metal and decreasing metal solubility by consuming ligands that are bound to the metal or by biosorption, enzymatic reduction, and precipitation, and by employing the metal as a micronutrient, as shown in Figure 1.
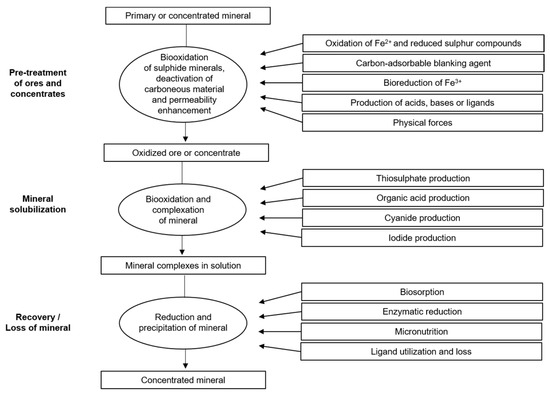
Figure 1. Possible roles of microorganisms in mineral processing and recovery. Source: Adapted with permission from Ref. [6]. 2007, Springer Nature.
As mentioned above, SMMC metal bioleaching processes can be carried out using bacteria or fungi; the decision-making for the selection depends on the objectives to be achieved in the bioprocess. Table 1 shows the general features for selecting the bacterial or fungal bioleaching application route.
Table 1. General features for the application of bacterial or fungal bioleaching. Source: Authors.
| Feature | Bacterial Bioleaching | Fungal Bioleaching |
|---|---|---|
| Application | Applied when it is required to recover a metal of interest, and it is not necessary to preserve the properties of the solid matrix | Applied when it is necessary to preserve the properties of the solid matrix, especially the crystalline properties, in case it is a mineral SMMC. |
| Applied if the final color of the solid matrix is not of interest. | ||
| Applied if not required to separate the fungus from the solid matrix. | ||
| Sterilization and Sanitization | Not required | Required |
| Stages | Performed in one step | Performed in two steps: Direct method (See Section 4.2). |
| Performed in two steps: Indirect method (See Section 4.2). |
||
| Operational times | Prolonged due to bacteria acting directly in the process | Direct method: Simultaneous fermentation with leaching can take 3 to 10 days, depending on the target. |
| Process times depend on the SMMC/bacteria system | Indirect method: Production of the leaching solvents (fermented broth): can have a production time of 8 to 10 days, with possible constant production and storage for consumption, without this being the limiting stage. The bioleaching process takes approximately 4 to 6 h, depending on the solid matrix and the metal to be extracted. |
Bacterial bioleaching is applied when the recovery of a metal of interest is required, and it is not necessary to preserve the properties of the solid matrix. On the other hand, fungal bioleaching is applied when it is necessary to preserve the properties of the solid matrix, especially the crystalline properties, in case it is a mineral SMMC. Furthermore, sterilization and sanitization processes are required in fungal but not bacterial bioleaching due to their resistance to contamination, and because the process is usually performed in one step, which limits the exposure to external contaminants. In contrast, fungal bioleaching is performed in two steps, either by a direct or indirect method.
The operational times differ between the two methods, with bacterial bioleaching being prolonged due to the bacteria acting directly in the process. In contrast, fungal bioleaching has variable times depending on the SMMC/bacteria system and the method used. The indirect method of fungal bioleaching has a longer production time for the leaching solvents (fermented broth) but has a shorter bioleaching process time. The information provided in this table can assist researchers and industry professionals in selecting the appropriate bioleaching method according to their specific metal recovery requirements and constraints.
2. Bacterial Bioleaching
Bacterial leaching, also known as bioleaching, biohydro-metallurgy, or bio-oxidation of sulfides, can be defined as a natural dissolution process resulting from the action of a group of bacteria, mainly of the genus Thiobacillus, with the ability to oxidize sulfide SMMC, allowing the release of the metals contained in them [8][9].
Bacterial bioleaching is based on the ability of microorganisms to transform solid compounds into soluble and extractable elements [10] or expose metals contained in ores and concentrates by direct oxidation or indirect chemical oxidation caused by corrosive metabolic by-products generated by electrochemistry, or a combination of both [8].
The attack and solubilization of an SMMC through microorganisms are performed by different mechanisms, which depend on the matrix’s sulfur matrix [11][12].
Similarly, the bioleaching process occurs by the catalysis that microorganisms exert during the dissolution of certain SMMC; for example, microorganisms such as bacteria convert metal compounds into their water-soluble forms and are biocatalysts of these leaching processes [13][14]. The microorganism uses SMMC as fuel, electron transfer for its survival purposes, and releasing metals, without the need for external application of energy. In this type of process, high activation energies are not necessary; proof of this is that the reactions take place at low pressure and some at low temperature, depending on the type of microorganism, whereas other ways need extreme conditions for their development and performance [13].
According to Rodríguez et al. (2001), bacterial bioleaching or leaching is understood as the attack and solubilization of an SMMC through the direct or indirect action of different microorganisms [15].
Microorganisms adapted to bioleaching processes are of two categories:
- Autotrophic: These microorganisms obtain nutrients and energy for their life cycles from the inorganic matter surrounding them.
- Heterotrophic: They require the availability of organic matter to complete their life cycles.
In both groups or categories of bacteria, some species operate predominantly in the presence of oxygen. These are the aerobic bacteria that carry out, in the first place, the oxidation reactions and have the property of oxidizing metal sulfides to soluble sulfates. Similarly, anaerobic bacteria can function and carry out their life cycles without oxygen; these batteries first conduct reduction reactions [16]. The respective classification is shown in Figure 2.

Figure 2. The bacterial classification used in the bioleaching process.
According to Figure 2, regardless of the bacterial classification, the main characteristic of the microorganisms used in metal recovery is their capacity to evolve in environments with very aggressive or extreme temperatures, pH, and living conditions [13][15].
Some of the bacteria involved are acidophilic microorganisms capable of surviving at low pH levels, high temperatures, and high concentrations of metallic elements, and whose energy source is the oxidation of Fe2+ to Fe3+ and reduced sulfur compounds [15].
The most significant area within the biotechnological process of metals is focused on the microbial oxidation of sulfide SMMC. Low pH, high metal concentrations, and sometimes high temperatures characterize the aqueous environments associated with SMMC discharges. However, some microorganisms can live, develop and reproduce under these conditions and in these environments. In these environments, microorganisms use reduced sulfur species and certain metals in solution as a primary energy source, resulting in the solubilization of valuable metals [13][17].
According to Rodriguez et al. (2001), in recovery by bioleaching, the main action of microorganisms is direct oxidation through an enzymatic attack or indirect through regeneration of the leaching agent Fe3+ of SMMC [15].
In some cases, the bacteria contribute to the weathering of the gangue by liberating the valuable mineral and facilitating its subsequent attack since the sulfuric acid generated by the bacteria produces a rupture of the Si-O and Al-O bonds in the alumino-silicates of the mineral gangue [15].
The microorganisms used in the leaching of metals from ores and concentrates are presented in Table 2. These microorganisms are capable of oxidizing sulfides and sulfur or ferrous iron species. An oxidant (ferric sulfate) is formed when the latter is oxidized.
Table 2. Bacteria used in bioleaching and their optimum conditions.
| Microorganism | Energy Source | pH | T (°C) | References |
|---|---|---|---|---|
| Acidithiobacillus ferrooxidans | Ferrous iron, sulfide minerals, sulfur, thiosulfate | 1.7–3.5 | 28–30 | [18] |
| Leptospirillum ferrooxidans | Ferrous iron | 3.0 | 30 | [19] |
| Acidithiobacillus thiooxidans | Elemental sulfur, thiosulfate | 1.0–3.5 | 28–30 | [20] |
| Thiobacillus thioparus | Elemental sulfur, thiosulfate | 7.0–8.5 | 28–30 | [21] |
| Sulfobacillus thermosulfidoxidans | Ferrous iron, elemental sulfur, sulfide minerals | 2.1–2.5 | 50–55 | [22] |
| Sulfolobus acidocaldarius | Elemental sulfur, yeast extract | 2.0–3.0 | 70–75 | [23] |
| Sulfolobus brierly | Elemental sulfur, ferrous iron, yeast extract | 2.0–3.0 | 60 | [24] |
| Acidiphilium acidophilum | Elemental sulfur, thiosulfate, yeast extract, salts, sugars, amino acids | 2.0–3.0 | 28–30 | [24] |
The bioleaching of A. thiooxidans and A. ferrooxidans contributes via both “contact” and “non-contact” mechanisms. The “contact” mechanism considers that most cells adhere to the surface of the bioleaching substrates. The “non-contact” mechanism is related to redox reactions, such as iron (III) reduction and sulfur oxidation [17][25].
In this sense, there are many and varied definitions of bioleaching. However, what should be made clear is that this leaching can be determined because the raw material studied can be under the direct or indirect action of microorganisms [26].
According to Table 2, a direct mechanism is understood as that which is mediated by bacterial action and where the chemical reactions are catalyzed enzymatically; this option also supposes the physical contact of the microorganisms with the solid matrix [26].
On the other hand, an indirect mechanism involves chemical reactions, enzymatic or non-enzymatic, with no physical contact between the microorganisms and the solid matrix. However, the microorganisms are relevant in forming chemical reagents that can participate in the process [26][27].
The first attempt to explain the mechanism of bioleaching was made by Silverman and Ehrlich in 1964 when they proposed two possible mechanisms [28], as shown in Figure 3.
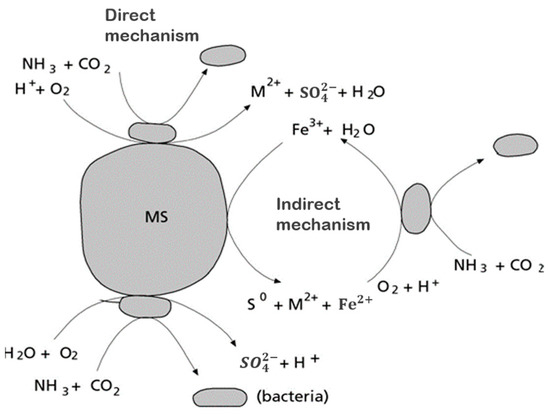
Figure 3. Direct and indirect mechanisms of bioleaching. Adapted from [28].
According to the first possible mechanism, the bacteria attack the metal sulfide directly by adhering to the mineral surface and then enzymatically oxidize it by transporting electrons from the reduced part of the mineral, usually a sulfide, to dissolved oxygen. The general reaction is presented in Equations (1) and (2) [4][10][14].
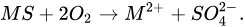 (1)
(1)
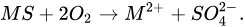 (1)
(1)In the case of pyrite, the above reaction would look like this:
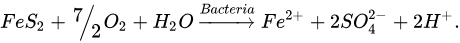 (2)
(2)
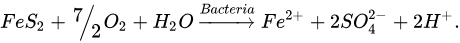 (2)
(2)The first mechanism proposed (Equation (1)) states that the oxidation of the mineral is carried out by direct enzymatic action, in which the role of the microorganisms is to catalyze the metal sulfide dissolution reaction. The iron-oxidizing bacteria can leach metal sulfides directly without the participation of biologically produced ferric sulfate. The M represents a bivalent metal. The second mechanism, called indirect (Equation (2)), is based on microorganisms oxidizing the minerals by generating ferric ion. In the case of pyrite, the direct transformation of sulfide to sulfate occurs through enzymatic oxidation, where the ferrous sulfate formed is oxidized to ferric sulfate in a later stage by the bacteria [19].
This theory of direct mechanism has been supported by several experimental studies that confirm the adherence of bioleaching bacteria to the surface of minerals. The work presented by Berry and Murr evidence that Acidithiobacillius spp. secrete some substances that can help the attack, although their nature is unknown [29].
Similarly, pyrite crystals exposed to the action of A. ferrooxidans were observed under the scanning electron microscope, detecting traces of corrosion in the crystals and suggesting that the bacteria caused these through a metabolite that could act in three different ways: oxidation of the ferrous ion to ferric, solubilization of the sulfur on the surface of the mineral or by direct attack. In addition, it was found that A. ferrooxidans could discern the most favorable regions of the mineral surface to obtain its energy source and select the site of attack according to the best availability of nutrients. However, the authors, far from using these results in favor of the direct mechanism, have used them to explain the indirect mechanism known as contact leaching [30].
Studies carried out by different authors also detected by electron microscopy the formation of holes on the surface of dissolved sphalerite in the presence of bacteria, and it was concluded that they occurred due to the direct attack of the bacteria on the surface of the mineral [31]. Tributsch studied by SEM the surface of lead sulfide after bacterial attack, showing depressions where the bacteria had been located. This author concluded that the bacteria produced a chemical carrier that promoted the attack at the point of adhesion of the microorganisms [32].
Studies by some authors mention that bacteria breathe using minerals, which provides a new vision of the problem, although, in this case, not related to sulfide minerals [33][34].
In contrast to the direct mechanism, the indirect mechanism considers the action of ferric ions on the sulfide ore dissolving it. Through this chemical leaching reaction, ferrous ions and elemental sulfur are produced. Finally, these chemical species are biologically oxidized to ferric iron and sulfate ions. This mechanism, in principle, does not require the adherence of cells to the sulfide mineral [26][34].
Some of the bacteria mentioned in Table 3 show extraction efficiencies in laboratory experiments with SMMC, such as minerals, sediments contaminated with metals after wastewater treatment, and printed circuit boards, among other materials that can be returned to the production chain once they fulfill their useful life and become waste.
Table 3. Bacterial bioleaching: operating conditions and metal extraction efficiencies of different SMMCs.
| Microorganism | SMMC | Metal | pH | T (°C) | Agitation | Pulp Density | Efficiencies | References |
|---|---|---|---|---|---|---|---|---|
| A. ferrooxidans, Desulfotomaculum geothermicum | Crushed and screened graphitic schist with a diameter of 8 mm | Iron, zinc, nickel, copper, and cobalt | 1.7–2.0 | 40–50 | - | - | In 500 days, the recoveries were Ni 92%, Zn 82%, Co 14%, and Cu 2%. | [35] |
| A. ferrooxidans | Pyrrhotite, chalcopyrite and arsenopyrite | Iron, copper | 2.8–3.2 | – | - | - | In 41 days, recoveries were 47.4 mg/L at a pH of 3.2. | [36] |
| A. ferrooxidans | Dried and crushed sludge at different particle sizes | Gold, copper, zinc, lead | 1.8–2.2 | 30 | 100 rpm | 6.0% (w/v) | In 14 days, the extractions were 4.71%, 9.01% Pb, 12.98% Cu, and 31.88% Zn. | [25] |
| A. ferrooxidans | Quartz, chlorite, chalcopyrite, albite, pyrite | Aluminum, iron, copper | 1.8 | 30 | 150 rpm | - | In 5 weeks, metal recoveries were 47.29% Al, 54.41% Fe, and 28.08% Cu. | [37] |
| A. ferrooxidans FT-22, A. ferrooxidans FT-23, A. ferrooxidans BF, and A. ferrivorans | Albite, quartz, clinochlore, muscovite, illite | Silver, copper | 10.5–11.0 | 25 | 20–30 rpm | 40% (w/v) | In 48 h, the recovery of metals was 51% Ag and 70% Cu. | [38] |
| Sulfobacillus thermosulfidooxidans, A. thiooxidans, Acidiphilum multivorum, and Leptospirillum Ferriphilum. | Chalcopyrite | Iron, copper | 2.0 | 30 | - | 1–6% (w/v) | In 11 days, the metal recovery was 28.57% Fe and 39.55% Cu. | [39] |
| S. Thermosulfidooxidans, A. thiooxidans/A. ferrooxidans, S. thermotolerans, and A. albertensis. | Clay, sand, silt | Zinc, copper, nickel, chromium | 1.5–3.1 | 30 | 200 rpm | 10% (w/v) | In 20 days of operation, metal recovery was 49% Zn, 50% Cu, 65% Ni and 27% Cr. | [40] |
| Sulfobacillus thermophidus oxidans | Printed circuit board (PCB) | Aluminum, lead, zinc, and tin | - | 45 | 120–145 rpm | 0.33% (w/v) | Recovery of 83% Zn, 89% Cu, and 81% Ni in 18 days. | [41] |
| A. thiooxidans and A. ferrooxidans | Soil contaminated with metals and metalloids | Cadmium, copper, lead, zinc, zinc, chromium, iron | 5.6 | 30 | 150 rpm | 10% (w/v) | In 42 days, metal recovery was 36% Fe and 70% Zn. | [42] |
| Burkholderia spp. Z-90 | Soil contaminated with metals and metalloids | Cadmium, arsenic, copper, lead, lead, zinc, chromium, iron | 3.0 | 35 | 180 rpm | 5% (w/v) | In 5 days, the maximum metal recovery achieved was 31.6% As, 37.7% Cd, 24.1% Cu, 52.2% Mn, 32.5% Pb, and 44% Zn. | [43] |
| Shewanella putrefaciens | Soil contaminated with metals and metalloids | Cadmium, arsenic, copper, lead, lead, zinc, chromium, iron | 2.2 | 30 | 100 rpm | 3% (w/v) | Arsenic recovery was 57.5% in 40 days. | [44] |
| Acidithiobacillus, Acetobacter, Acidophilum, Acidophilum, Arthrobacter spp., and Pseudomonas spp. | Panchakavya (soil mixture) | Cadmium, arsenic, copper, lead, lead, zinc, chromium, iron | 2.6 | 30 | 120–180 rpm | 0.2–1% (w/v) | Metal recovery in 5 days was 64% Pb and 49% Cu. | [45] |
| Massilia spp., Alicyclobacillus spp., and Micromonospora spp. | Soil contaminated with metals and metalloids | Cadmium, arsenic, copper, lead, lead, zinc, chromium, iron | 3.5 | 30 | 180 rpm | 1% (w/v) | The metal extraction in 10 min was 32.09% Cd | [46] |
| Myxotrophic acidophiles | Soil contaminated with metals and metalloids | Cadmium, arsenic, copper, lead, lead, zinc, chromium, iron | 2.0 | 25 | 175 rpm | 4% (w/v) | In 14 days, the two-step bioleaching achieved the extraction of 34% Cd | [47] |
| Indigenous bacteria | Agricultural land | Zinc, copper, nickel | 8.0 | 28 | 180 rpm | 1% (w/v) | In 9 days, the maximum metal extraction achieved was 74.72% Cu, 35.35% Ni, and 69.92% Zn. | [48] |
| A. ferrooxidans, A. thiooxidans, and L. ferriphilum | Pyrite and sulfosalts | Aluminum, manganese, iron, copper, zinc, mercury, zinc, mercury | 4.0 | 30 | 180 rpm | 5% (w/v) | In 31 days, the maximum metal recovery was 93.3% Cu, 92.13% Mn, and 96.1% Zn. | [49] |
| Sulfobacillus thermosulfidooxides and A. caldus | Pyrite and sulfosalts | Aluminum, manganese, iron, copper, zinc, mercury, zinc, mercury | 7.5 | 45 | 180 rpm | 5% (w/v) | Fermentation was carried out for 31 days, and the metal recovery efficiency was 45% As, 89% Cd, 94% Cu, 34% Hg, 95% Mn, and 98% Zn. | [49] |
| Indigenous bacteria | Port sediments | Copper, chromium, cadmium, lead, zinc | 6.0 | 30 | 100 rpm | 1% (w/v) | During 30 days of processing, the recovery of metals was 29% Cu, 8% Ni, 5% Pb, and 39% Zn. | [50] |
| Bacteria from exogenous soil | Port sediments | Copper, chromium, cadmium, lead, zinc | 8.0 | 30 | 100 rpm | 4% (w/v) | During 30 days of processing, the recovery of metals was 100% Cu, 95% Cr, 100% Ni, 100% Pb, 100% Zn, 100% Cu, 95% Cr, 100% Ni, 100% Ni, 100% Pb, 100% Pb and 100% Zn. | [50] |
| A. ferrooxidans and A. thiooxidans | Anaerobic sediment from urban wastewater | Copper, chromium, cadmium, lead, zinc | 5.0 | 25 | 120 rpm | 15% (w/v) | Metal recovery during 57 days was 43% Cu and 80% Zn. | [51] |
| A. ferrooxidans, A. thiooxidans, and Leptospirillum ferriphilum | Sediment from sewage outfall | Copper, chromium, cadmium, lead, zinc | 4.0 | 30 | 180 rpm | 5% (w/v) | Metal recovery was 90.9% Cu and 94.74% Zn; elements such as Cd, Hg, Mn, and Pb were below 30%. | [52] |
| A. ferrooxidans | Mining tailings | Copper, iron, cadmium, antimony, zinc, nickel, chromium, nickel, chromium | 3.0 | 30 | 200 rpm | 5% (w/v) | In 20 days, the maximum efficiency achieved was 36.2% Cu, 65.95% Cr, 97.4% Ni, 2.2% Sb, and 34.8% Zn. | [53] |
| A. ferrooxidans and A. thiooxidans | Mining tailings | Arsenic, zinc, copper, lead | 2.5 | 30 | 200 rpm | 5% (w/v) | Metal recovery in 25 days was 72.2% As, 47.1% Cu, 99.5% Mn, and 78.9% Zn. | [53] |
| A. ferrooxidans | Mining tailings | Arsenic, zinc, copper, lead | 2.0 | 30 | 200 rpm | 20% (w/v) | The maximum metal recovery achieved in 50 days was 71.37% Fe, 0.82% Pb, and 97.38% Zn. | [54][55] |
| A. thiooxidans | Tailings from an abandoned and inactive mine | Arsenic, zinc, copper, lead | 1.8 | 40 | 150 rpm | 0.5% (w/v) | Arsenic recovery in 25 days was 47%. | [53][56][57] |
| A. ferrooxidans | Mine and metallurgical wastes | Lead, iron, copper, zinc | 1.5 | 40 | 160 rpm | 20% (w/v) | In 50 days of fermentation, the metal recovery achieved was 85.45% Fe, 4.12% Pb, and 97.85% Zn. | [54][55] |
| A. ferrooxidans and A. thiooxidans | Mine tailings deposits | Silver, lead, mercury, zinc, zinc, arsenic, manganese, indium, gallium, germanium, cobalt | 6.0 | 30 | 200 rpm | 5%(w/v) | In 20.8 days, 42.4% As, 45% Cu, 47.7% Fe, 92% Mn, and 67.2% Zn were extracted. | [53][56] |
| A. ferrooxidans and A. thiooxidans | Mine waste | Arsenic, manganese | 2.5 | 30 | 200 rpm | 5% (w/v) | In 35 days, metal recovery was 96.7% As and 100% Mn. | [20] |
| Leptospirillum ferriphilum, A. caldus, Sulfobacillus thermosulfidooxidan, A. sulfuroxidans, Ferroplasma acidiphilum, Acidiplasma sp., Sulfobacillus acidophilus, Acidithiobacillus spp., and Acidiphilum cryptum. | Mining waste materials | Silver, lead mercury, zinc, arsenic, manganese, indium, gallium, germanium, and cobalt. | 1.7 | 45 | 150 rpm | 5% (w/v) | Bioleaching was carried out for 50 days, and the efficiency of recovered metals was 90% Cu and 99% Zn. | [58] |
| Leptospirillum ferriphilum, A. caldus, Sulfobacillus sp. and Ferroplasma sp. | Mine tailings deposits | Silver, lead, mercury, zinc, zinc, arsenic, manganese, indium, gallium, germanium, cobalt | 1.8 | 45 | 150 rpm | 5% (w/v) | In 30 days, the metal recovery was 59.5% Co, 55% Cu, and 98.2% Ni. | [59] |
| Leptospirillum ferriphilum, A. caldus, Sulfobacillus sp. and Ferroplasma sp. | Mine tailings deposits | Silver, lead, mercury, zinc, zinc, arsenic, manganese, indium, gallium, germanium, cobalt | 1.2 | 45 | 150 rpm | 5% (w/v) | In 30 days, the metal recovery was 36.5% Co, 72% Cu, and 61.2% Ni. | [59] |
| A. caldus, Leptospirillum ferriphilum, Methylophaga spp. and Sphingomonas spp. | Tailing material in mining areas in Germany | Silver, lead, mercury, zinc, zinc, arsenic, manganese, indium, gallium, germanium, cobalt | 1.5 | 40 | 550 rpm Aeration: 5 L/min | 15% (w/v) | Fermentation was carried out for seven days, and the metal extraction achieved was 105,000 mg/kg. | [18] |
| A. caldus and Leptospirillum ferriphilum | Scorodite | Arsenic, copper, iron, sulfur | 1.2 | 45 | Aeration: 200 mL/min | 1% (w/v) | The maximum recovery achieved during 88 days was 97% As. | [60] |
| Acidophilic ferrous, iron-oxidizing, and sulfur-oxidizing species | Tailing material in mining areas in Germany | Silver, lead, mercury, zinc, zinc, arsenic, manganese, indium, gallium, germanium, cobalt | 1.6 | 30 | 100 rpm | 4% (w/v) | During 22 days, the extraction efficiencies reported were 100% As, 85% Cd, 40% Cu, 85.4% Ln, 100% Mn, 5% Pb, and 100% Zn. | [61] |
| Acidophilic ferrous iron-oxidizing and sulfur-oxidizing species | Tailing material in mining areas in Germany | Silver, lead, mercury, zinc, zinc, arsenic, manganese, indium, gallium, germanium, cobalt | 1.8 | 30 | 100 rpm | 4% (w/v) | During 22 days, the reported extraction efficiencies were 79.9% In and 94.6% Zn. | [61] |
| Acidophilic ferrous iron-oxidizing and sulfur-oxidizing species | Tailing material in mining areas in Germany | Silver, lead, mercury, zinc, zinc, arsenic, manganese, indium gallium, germanium, cobalt | 1.8 | 30 | 100 rpm | 10% (w/v) | During 22 days, the reported extraction efficiencies were 72% As, 88% Cd, 87% In, and 81% Zn. | [61] |
| A. thiooxidans Ram 8, A. ferrooxidans Ram 6F, Leptospirillum ferrooxidans, and Ferroplasma acidiphilum BRGM 4 | Tailing and mining residues (pyrite, quartz, etc.) | Iron, zinc, silica, cobalt, cobalt, nickel, aluminum, manganese, arsenic | 2.0 | 30 | 150 rpm | 10% (w/v) | The recovery achieved in the fermentative process was 91% Co, 57% Cu. | [62] |
| Marinobacter sp., Acidithiobacillus spp., Leptospirillum sp., Cuniculiplasma sp., Nitrosotenius sp. and Ferroplasma sp. | Tailing and mining residues (pyrite, quartz, etc.) | Iron, zinc, silica, cobalt, cobalt, nickel, aluminum, manganese, arsenic | 1.5 | 30 | 300 rpm | 10% (w/v) | In 10 days of retention, the amount of metal recovered was 87% Co, 43% Cu, 67% Ni, and 100% Zn. | [63] |
| Leptospirillum ferriphilum YSK, Ferroplasma thermophilum L1, A. caldus S1, and A. thiooxidans A01. | Metallurgical industry waste | Copper, cobalt, nickel, zinc | 1.8 | 40 | 175 rpm | 5% (w/v) | In 16 days, the maximum copper recovery was 58.7%. | [64] |
| Indigenous bacterial and fungal strains | Mining waste | Silver, manganese | 2.0 | 30 | 200 rpm | 6% (w/v) | 67% Ag, 745 Mn. | [65] |
3. Fungal Bioleaching
Leaching by fungal action is called fungal bioleaching. It occurs mainly by the action of organic acids produced in the fermentative process [66] when metals are mobilized from solid materials through ligand-induced metal solubilization [67].
The fungi used in producing organic acids, such as citric, oxalic, gluconic, and others, are shown in Table 4. They can be quickly grown using unsophisticated fermentation techniques and inexpensive growth media [68].
Table 4. Organic acids produced by selected fungus in fermentative processes.
| Fungi | Organic Acids |
|---|---|
| Yarrowia lipolytica | Citric acid |
| Mucor spp. | Fumaric and gluconic acid |
| Rhizopus spp. | Lactic, fumaric and gluconic acid |
| Aspergillus niger | Citric, oxalic and gluconic acids |
| Aspergillus spp. | Citric, malic, tartaric, ketoglutaric, itaconic and aconitic acid |
| Penicillium spp. | Citric, malic, tartaric, ketoglutaric, ketoglutaric and gluconic acids |
| Schizophyllum commune | Malic acid |
| Paecilomyces variotii | Malic acid |
In the context of fungal bioleaching processes, it is important to note that while fungi can produce mycotoxins that pose risks in food matrices, these concerns are not typically relevant in industrial processes such as bioleaching. Fungal mycotoxins have been known to cause issues ranging from crop yield reductions and negative impacts on farm animal health to alterations in food quality, organoleptic characteristics, and nutritional value. The associated costs include prevention and decontamination measures [69].
Human exposure to mycotoxins primarily occurs through food consumption, although isolated cases of respiratory-related ailments have been reported. Since food represents the main source of risk for humans, it justifies the inclusion of mycotoxins as one of the molecule groups considered in the Food Safety program. However, in the context of industrial processes like bioleaching, the focus is primarily on harnessing the beneficial capabilities of fungi without significant concern for mycotoxin-related issues, as these processes involve different considerations and control measures [69][70][71].
Fungal cells have a specific structure or organization in the material, where various components are distinguished, such as cell wall, plasma membrane, cytoplasm, mitochondria, endoplasmic reticulum and the Golgi apparatus. Along with its structure appears the function of the cell, which is basically to remain viable, develop and multiply. This function of the cell is mediated by the various components that constitute it [68].
Studies by different authors reveal that the filamentous fungus Penicillium simplicissimum (same as Penicillium janthinellum) is one of the most versatile microorganisms for the biotechnological production of organic acids due to its adaptability to different culture media and its selectivity at a metabolic level to generate multiple organic acids.
Other filamentous fungi such as Aspergillus spp., Fusarium graminearum, Aspergillus fumigatus, and Trichoderma harzianum also produce organic acids [68][72][73][74], mainly by submerged fermentation using different carbon sources (substrates) such as cellulosic waste hydrolysates, cane molasses, glycerol, and even primary alcohols.
There are also solid-state fermentation processes that simultaneously obtain citric and gluconic acid using agro-industrial residues as substrates [75]. However, submerged fermentation systems are preferred to produce the different acids since they allow better control of the process and, therefore, greater productivity of these acids [75][76].
The fungal bioleaching process is carried out by direct or indirect methods, as shown in Figure 4.
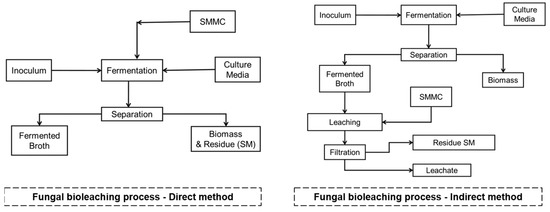
Figure 4. Fungal bioleaching process. Source: the authors.
Direct method: this method is carried out in one stage where the fermentation occurs: SMMC/culture medium/inoculum (fungus) simultaneously. In the process, the fungus produces the leaching substances, mainly organic acids, and simultaneously extracts the metal contained in the SMMC. The process can take 3 to 10 days depending on the objective, and the pH value of the fermentation usually starts between 6 and 7. As the bioprocess progresses, it reaches pH values between 3.5 and 4, which leads to a decrease in the efficiency of the process because the fungus is inhibited by the presence of SMMC residues [68].
Indirect method: this approach is carried out in two stages [68].
Stage 1: Production of the solvent extractor or fermented broth. Fermentation: culture medium/inoculum (fungus) for 8–10 days. Subsequently, a separation process is carried out, where the biomass is discarded, and the focus is on the fermented broth. This stage generally presents pH values below 3.0.
Stage 2: The fermented broth is in contact with the SMMC, a process that can take 4 to 8 h depending on the SMMC. Subsequently, a separation process is carried out, where the metal-free solid residues are discarded and the focus is on the fermented broth containing the extracted metals (leachates).
Acids are excellent metal leachants from SMMC. Heterotrophic metabolism can also achieve leaching due to the excretion of siderophores and organic acids. These provide protons and metal complexing anions, as citrate and oxalate anions can form stable complexes with many metals [68], including chelates which are relevant in metal dissolution by acidolysis and complex formation mechanisms. In conclusion, the main mechanisms through which organic acids interact with metals (bioleaching reactions) are acidolysis (acid-base reaction), redoxolysis, bioaccumulation, chelates, and complex formation mechanisms [3].
The efficiency of the process depends on the acids produced and their acidity depending on their functional group (e.g., carboxylic or sulfonic). Considering that these acids are not equal in the number of carboxylic groups, hydroxyl groups, and carbon–carbon double bonds in their molecules, they are classified according to their typical characteristics of the carbon chain, saturation, substitution, and functional groups.
Based on the Lewis acid-base theory, the ability to release protons is shown by the strength of the acid. The stronger the acidity, the stronger the ability to release protons. For example, oxalic acid (pKa 1.23) shows higher acidic capacity than formic acid (pKa 3.75), implying that the former has higher leaching power provided that the conjugate base of the oxalate anion does not interact strongly with the metal of interest, causing the precipitation of metal complexes [79].
Studies show that oxalic acid (pKa 1.23) leached more iron ions than lactic acid (pKa 3.86). The pKa values of each carboxylic acid indicate that oxalic acid is stronger than lactic acid, which leaches metals more efficiently. In addition, the complexation reaction and the formation of iron oxalates may play a relevant role in increasing the leaching of iron ions, which is confirmed in the leaching of iron contained in kaolin using oxalic acid, which is more effective than when organic acids such as citric, malonic, or acetic acid are used [80]. Oxalates are known for chelating metals, and this can be exploited for the dissolution and separation of metals. The increase in the solubility of simple oxalate compounds in the presence of excess oxalate provides the basis for formation of the oxalate complex [81].
Metallic oxalates display a diverse range of solubilities which are influenced by the particular metal cation and solution conditions. Insoluble oxalates include calcium oxalate (CaC2O4), silver oxalate (Ag2C2O4), and lead oxalate (PbC2O4). Conversely, sodium oxalate (Na2C2O4) and potassium oxalate (K2C2O4) exhibit high solubility, while barium oxalate (BaC2O4) is generally soluble. Transition metal oxalates like iron oxalate (FeC2O4) and nickel oxalate (NiC2O4) can display variable solubility depending on conditions. Factors such as pH, temperature, presence of other ions, and ligand complexation influence the solubility behavior of metallic oxalates [81].
In bioleaching processes, the binding behavior of oxalic acid is influenced by adsorption of oxalic acid onto metal surface which leads to the formation of an upright mono-oxalate species, with the carboxylate group binding to the surface. This upright orientation enables interactions between the carboxylic acid group and the surrounding environment, resulting in the creation of a chemically functionalized surface. The presence or absence of intramolecular hydrogen bonding determines the various surface species of mono-oxalate. The tilting of the molecules is influenced by surface coverage and temperature, leading to the observation of both unidentate and bridging species [82].
L-aspartic acid can release a total of three protons: two protons from its carboxyl groups and one proton from its amino group. On the other hand, L-tartaric acid does not possess an amino group, and therefore, two acidity constants are reported specifically for its carboxyl groups. In the case of L-glutamic acid, two protons are released from its carboxyl groups, while an additional proton is released from its amino group. Glutamate and aspartate chelate metal ions weakly via the amino nitrogen and carbonyl oxygen bind. A stronger chelation occurs upon amide-nitrogen-bound hydrogen by some metal ions such as Cu2+. This reaction occurs in neutral pH conditions (pH ≈ 7) with Cu2+ [83].
Several reports have documented the leaching of metals with fungi. Fungi of the genera Aspergillus and Penicillium are among the most effective and relevant for biological leaching due to filamentous fungi’s robust adaptability and ability to tolerate metal contamination stress [3].
Laboratory studies have been carried out using fungi, where only the leaching action is specified according to the type and species of microorganism. For example, A. niger, P. simplicissimum, and P. purpurogenum are related to the production of low-molecular-weight metabolites, mainly organic acids such as gluconic acid, pyruvic acid, citric acid, oxalic acid, malic acid, and succinic acid, different from the mechanism reported for R. rubra, A. thiooxidans and A. ferrooxidans [68].
Some of the most common organisms for fungal bioleaching are the genera Acidithiobacillus, Aspergillus, and Penicillium. For example, Penicillium simplicissimum is used to extract elemental Zn from ZnO. Fungi are also particularly suitable for phosphorus bioleaching, where organic acids such as citric and oxalic acid solubilize P from Fe and Al phosphates. In this process, carboxylic anions compete with binding sites that chelate Al3+ and Fe3+ [67].
Many microorganisms are reported to solubilize metals in soils, including Aspergillus niger, Penicillium purpurogenum, Rhodotorula rubra, Acidithiobacillus thiooxidans, and Acidithiobacillus ferrooxidans. The mechanism of metal solubilization during the process is related to a chemical process where the binding of microorganisms to the mineral can enhance dissolution [14][84].
Despite successful records of fungal tolerance to metals at the laboratory scale, no documentation of their use at the industrial scale is available [3][85].
In Table 5, fungal bioleaching experiments are presented: operating conditions and metal extraction efficiencies of different SMMC.
Table 5. Fungal bioleaching: operating conditions and metal extraction efficiencies of different SMMCs.
| Microorganism | SMMC | Metal | pH | T (°C) | Agitation-Aeration | Pulp Density | Result | References |
|---|---|---|---|---|---|---|---|---|
| Aspergillus niger | Spent FCC catalyst (zeolites), crushed and screened | Nickel, vanadium, aluminum, aluminum, antimony, molybdenum, cobalt, tungsten | 6.0 | 30 | - | 1% (w/v) | In 60 days, the recovery was 9% Ni, 23% Fe, 30% Al, 36% V, and 64% Sb. | [86] |
| Penicillium simplicissimum | Spent FCC catalyst (zeolites), crushed and screened | Nickel, vanadium, aluminum, aluminum, antimony, molybdenum, cobalt, tungsten | 4–7 | 30 | - | 3% (w/v) | In two-step bioleaching, 32% Al, 67% Co, 65% Mo, and 38% Ni were recovered in 30 days. | [87] |
| Purpureocillium lilacinum y Aspergillus niger (7:3) | Printed circuit boards, crushed with d < 40mm | Aluminum, lead, zinc, and tin | 5.0 | 30 | 150 rpm | 3 to 8% (w/v) | In 27 days, 15.7 ± 0.87% Al, 20.5 ± 0.78% Pb, 49.5 ± 0.38% Zn and 8.1 ± 0.34% Sn were extracted. | [88] |
| Aspergillus niger | Printed circuit boards, shredded and screened | Aluminum, lead, zinc, copper | 5.08 | 25 | 120 rpm | 3.9% (w/v) | In 21 days, the maximum recovery of metals was 98.57% Zn, 43.95% Ni, and 64.03% Cu. | [89] |
| P. simplicissimum | Printed circuit boards | Aluminum, lead, zinc, copper | 6.0 | 30 | 100–400 mL/min | 1–10% (w/v) | The maximum recovery achieved for Cu and Ni was 40% in 7 days. | [90] |
| Aspergillus niger | Saprolite | Iron, silica, nickel, manganese | 5.0 | 95 | 400 rpm | 10% (w/v) | The maximum recovery achieved in 24h was 65% Ni and 58% Fe. | [91] |
| Aspergillus niger | Limonite | Iron, aluminum, silica, manganese | 5.0 | 95 | 400 rpm | 10% (w/v) | Maximum recovery achieved in 24h was 78% Ni and 60% Fe. | [91] |
| Penicillium simplicissimum | Waste ash from power plant | Vanadium, iron, nickel | 4.5 | 30 | 130 rpm | 1% (w/v) | The maximum extraction achieved was 48.3% Fe, 19% V, and 12% Ni in 15 days. | [92] |
| Aspergillus niger NCIM 548 | Chromite | Nickel, cobalt | 2.5 | 30 | 150 rpm | 2% (w/v) | In 21 days of fermentation, the metal recovery was 70.49% Ni and 66.93% Co. | [93] |
| Aspergillus niger | Fly ash from municipal solid waste incinerators | Aluminum, lead, zinc, copper | 10–12 | 30 | 120 rpm | 1–8% (w/v) | After 30 days, the recovery of Cu, Pb, and Fe metals was between 60 to 70%, 55 to 70%, and 30 to 40%, respectively. | [94] |
| Aspergillus niger y Aspergillus tubingensis | Electronic waste (e-waste) | Copper, lead, tin, silver, gold, platinum, platinum, aluminum, manganese, and palladium | 5.0 | 30 | 140 rpm | 1%(w/v) | The achieved metal recovery was 80% Al, 50% Co, 90% Mn, 80% Li and 67% Ni in 27 days. | [95] |
| Aspergillus niger | Bauxite (d < 180 µm) | Aluminum, iron, silica | 6.5 | 30 | 130 rpm | 1% (w/v) | Metal recovery in 10 days was 82.80% Al. | [96] |
| Aspergillus niger adaptado | Lithium-ion batteries (LIBs) | Cobalt, lithium, nickel, manganese, copper, aluminum, graphite, and other materials | 5.4 | 30 | 120–170 rpm | 0.3–1% (w/v) | The obtained recovery efficiency from spent LIBs was 100%, 94%, 72%, 62%, 45%, and 38% for Li, Cu, Mn, Al, Ni, and Co, respectively, in 27 days. | [97] |
| Penicillium chrysogenum strain F1 | Soil contaminated with metals and metalloids | Cadmium, copper, lead, zinc | - | 25 | 120 rpm | 5% (w/v) | In 15 days, the recovery of metals was 50% Cd, 35% Cu, 9% Pb, and 40% Zn. | [84] |
| Phanerochaete chrysosporium | Waste of electrical and electronic equipment | Copper, lead, tin, silver, gold, platinum, platinum, aluminum, manganese, and palladium | 5.0 | 30 | 150 rpm | 1% (w/v) | In 14 days, the copper recovery achieved was 54%. | [79] |
| Aspergillus fumigatus (M3Ai) |
Soil contaminated with metals and metalloids | Cadmium, copper, lead, lead, zinc, chromium | 6.5 | 30 | 130 rpm | 5% (w/v) | In 3 days, the metal recovery in two-step bioleaching was 79% Cd and 69% Cr. | [98] |
| Aspergillus flavus | Soil contaminated with metals and metalloids | Cadmium, copper, lead, lead, zinc, chromium | - | 30 | 130 rpm | 5%(w/v) | In 15 days, the maximum metal recovery was 39.77% Cd, 18.16% Pb, and 58.22% Zn. | [50] |
| Fibroporia vaillantii | Wood preservative: Chromated copper arsenate | Chromium, copper, arsenic | 3.1 | 30 | 150 rpm | - | In 28 days of fermentation, the maximum metal recovery efficiency was 87% Cu, 80% Cr, and 100% As. | [99] |
| Geotrichum sp. G1 y Bacillus sp. B2 | Soil contaminated with metals and metalloids | Cadmium, copper, lead, lead, zinc, chromium | 2.0–10 | - | - | 2% | Chromium extraction at 28 days was 94.8%. | [100] |
| Aspergillus niger (M1DGR) |
Soil contaminated with metals and metalloids | Cadmium, copper, lead, lead, zinc, chromium | 6.5 | 30 | 130 rpm | 5% (w/v) | The 3-day two-step bioleaching metal recovery was 98% Cd and 43% Cr. | [98] |
| Penicillium rubens (M2Aiii) |
Soil contaminated with metals and metalloids | Cadmium, copper, lead, lead, zinc, chromium | 6.5 | 30 | 130 rpm | 5% (w/v) | The 3-day metal recovery in two-step bioleaching was 79% Cd and 69% Cr. | [98] |
| Penicillium, Aspergillus, y Fusarium |
Panchakavya (soil mixture) | Cadmium, arsenic, copper, lead, lead, zinc, chromium, iron | 2.6 | 30 | 120–180 rpm | 0.2–1% (w/v) | The 5-day metal recovery was 64% Pb and 49% Cu. | [45] |
| Aspergillus niger strain SY1 | Contaminated sediment | Cadmium, arsenic, copper, lead, lead, zinc, chromium, iron | 6.5 | 30 | 220 rpm | 10% (w/v) | Metal recovery in 7 days was 93.5% Cd, 62.3% Cu, 11.5% Pb, and 68% Zn. | [101] |
| Aspergillus niger strain SY1 | Contaminated sediment | Cadmium, copper, lead, lead, zinc, chromium | 6.5 | 30 | 220 rpm | 2.5% (w/v) | In 15 days of fermentation, the recovery efficiency achieved was 90% Cd, 20% Pb, 60% Cu, and 60% Zn. | [101] |
| Penicillium chrysogenum strain KBS3 |
Mine tailings | Cobalt, zinc, copper, nickel, manganese, lead | 2.5 | 30 | 120 rpm | 10% (w/v) | In 25 days, the maximum metal recovery achieved was 60% Co, 67% Cu, 69% Mg, 55% Ni, and 65% Zn. | [65] |
| Aspergillus fumigatus | Mine tailings | Arsenic, iron, manganese, lead, zinc, zinc | 5.0 | 30 | 150 rpm | 8% (w/v) | In 40 days, the one-step bioleaching recovered 62.1% As, 58.4% Fe, 100% Mn, 56.1 Pb, and 54.43% Zn. | [102] |
| Aspergillus fumigatus | Mine tailings | Arsenic, iron, manganese, lead, zinc, zinc | 5.0 | 30 | 150 rpm | 8% (w/v) | The two-step bioleaching showed that the maximum metal recovery would be 32% As, 45.20% Fe, 58.4% Mn, 88.4% Pb, and 31.3% Zn. | [102] |
According to Table 5, the most important and significant area within the biotechnological process of metals is focused on the microbial oxidation of sulfide minerals. The aqueous environments associated with mine discharges and mining sites are typically characterized by low pH, high concentrations of heavy metals, and, in some cases, elevated temperatures. Despite these conditions and environments, certain microorganisms not only survive but also thrive and reproduce. In these environments, microorganisms utilize reduced sulfur species and certain metals in solution as their primary energy source, resulting in the solubilization of valuable metals [13][17].
Fungal species such as A. niger, P. simplicissimum, and P. purpurogenum are among the most used species in bioleaching and offer advantages over bacterial bioleaching, including the ability to thrive at high pH values and a faster leaching rate [84]. In these studies, pH is a control variable that indirectly indicates the presence or absence of organic acid production during the fermentation processes. pH influences the growth and excretion of organic acids in filamentous fungi. When grown in an unbuffered medium, filamentous fungi often rapidly acidify their environment to very low, and sometimes detrimental, pH values [103][104].
The production of organic acids and other metabolites in the fermentation process is also influenced by the agitation rate. Agitation at low to intermediate levels, ranging from 100 to 300 rpm, increases with agitation speed, whereas agitation speeds between 500 and 800 rpm further enhance it. Citrate synthase activity decreases with increasing agitation speed, while aconitase and isocitrate dehydrogenase activities increase with agitation speed, favoring the transformation of citrate into oxoglutarate [75].
Amino acids such as glycine, histidine, and alanine have been used to test their effect on gold solutions, revealing that the initial dissolution of gold in histidine solution is faster than in glycine and alanine solutions. However, upon extended leaching, it was found that glycine dissolves gold more rapidly and to a greater extent than histidine and alanine [105].
Pulp density plays a crucial role in determining the feasibility of applying bioleaching on a commercial scale. Increasing the pulp density from 1% to 2% (w/v) leads to a significant reduction of 50% in both the volume of leaching media required and the size of the bioreactor. This reduction in size and resource consumption directly translates into a substantial decrease in bioleaching costs. In the bio-hydrometallurgical treatment of low-grade ores, it is common to utilize pulp densities of 10% or higher to maximize efficiency and productivity [106].
This entry is adapted from the peer-reviewed paper 10.3390/su151310222
References
- Sousa, R.; Futuro, A.; Fiúza, A.; Vila, M.C.; Dinis, M.L. Bromine Leaching as an Alternative Method for Gold Dissolution. Miner. Eng. 2018, 118, 16–23.
- Brown, G.; Katz, D.; Foust, A. Unit Operations, 1st ed.; John Wiley & Sons: New York, NY, USA, 1960.
- Dusengemungu, L.; Kasali, G.; Gwanama, C.; Mubemba, B. Overview of Fungal Bioleaching of Metals. Environ. Adv. 2021, 5, 100083.
- Kaksonen, A.H.; Mudunuru, B.M.; Hackl, R. The Role of Microorganisms in Gold Processing and Recovery—A Review. Hydrometallurgy 2014, 142, 70–83.
- Latorre, M.; Cortés, M.P.; Travisany, D.; Di Genova, A.; Budinich, M.; Reyes-Jara, A.; Hödar, C.; González, M.; Parada, P.; Bobadilla-Fazzini, R.A.; et al. The Bioleaching Potential of a Bacterial Consortium. Bioresour. Technol. 2016, 218, 659–666.
- Reith, F.; Lengke, M.F.; Falconer, D.; Craw, D.; Southam, G. The Geomicrobiology of Gold. ISME J. 2007, 1, 567–584.
- Amachi, S. Microbial Contribution to Global Iodine Cycling: Volatilization, Accumulation, Reduction, Oxidation, and Sorption of Iodine. Microbes. Environ. 2008, 23, 269–276.
- Mahmoud, A.; Cézac, P.; Hoadley, A.F.A.; Contamine, F.; D’Hugues, P. A Review of Sulfide Minerals Microbially Assisted Leaching in Stirred Tank Reactors. Int. Biodeterior. Biodegrad. 2017, 119, 118–146.
- Lambert, F.; Gaydardzhiev, S.; Léonard, G.; Lewis, G.; Bareel, P.-F.; Bastin, D. Copper Leaching from Waste Electric Cables by Biohydrometallurgy. Miner. Eng. 2015, 76, 38–46.
- Potysz; Kierczak Prospective (Bio)Leaching of Historical Copper Slags as an Alternative to Their Disposal. Minerals 2019, 9, 542.
- Guerrero, L.; Esbrí, J. Aplicabilidad de la Biolixiviación Como un Método Sustitutivo de la Amalgamación con Mercurio para la Recuperación del Oro en la Minería Artesanal del Sur de Perú. Bachelor’s Thesis, Escola Politècnica Superior d’Enginyeria de Manresa, Lima, Peru, 2014.
- Khaing, S.Y.; Sugai, Y.; Sasaki, K. Gold Dissolution from Ore with Iodide-Oxidising Bacteria. Sci. Rep. 2019, 9, 4178.
- Ballester, A. Minería Química, 1st ed.; Instituto Tecnológico Geominero de España, Ed.; Instituto Tecnológico Geominero de España: Madrid, Spain, 1991.
- Mikoda, B.; Potysz, A.; Kmiecik, E. Bacterial Leaching of Critical Metal Values from Polish Copper Metallurgical Slags Using Acidithiobacillus thiooxidans. J. Environ. Manag. 2019, 236, 436–445.
- Rodríguez, Y.; Ballester, A.; Blázquez, M.L.; González, F.; Muñoz, J.A. Mecanismo de Biolixiviación de Sulfuros Metálicos. Rev. Metal. 2001, 37, 665–672.
- Chuquipoma, M.; Sergio, F. Biolixiviación; Osinergmin: Lima, Peru, 2016; Available online: https://www.osinergmin.gob.pe/seccion/centro_documental/mineria/Documentos/Publicaciones/Biolixiviacion.pdf (accessed on 10 May 2023).
- Zhang, S.; Yan, L.; Xing, W.; Chen, P.; Zhang, Y.; Wang, W. Acidithiobacillus ferrooxidans and Its Potential Application. Extremophiles 2018, 22, 563–579.
- Boon, M.; Snijder, M.; Hansford, G.S.; Heijnen, J.J. The Oxidation Kinetics of Zinc Sulphide with Thiobacillus ferrooxidans. Hydrometallurgy 1998, 48, 171–186.
- Sand, W.; Rohde, K.; Sobotke, B.; Zenneck, C. Evaluation of Leptospirillum Ferrooxidans for Leaching. Appl. Environ. Microbiol. 1992, 58, 85–92.
- Nguyen, V.K.; Ha, M.-G.; Shin, S.; Seo, M.; Jang, J.; Jo, S.; Kim, D.; Lee, S.; Jung, Y.; Kang, P.; et al. Electrochemical Effect on Bioleaching of Arsenic and Manganese from Tungsten Mine Wastes Using Acidithiobacillus spp. J. Environ. Manag. 2018, 223, 852–859.
- Sreekrishnan, T.R.; Tyagi, R.D. A Comparative Study of the Cost of Leaching out Heavy Metals from Sewage Sludges. Process Biochem. 1996, 31, 31–41.
- Bhattacharya, A.; Gupta, A. Current Trends in Applicability of Thermophiles and Thermozymes in Bioremediation of Environmental Pollutants. In Microbial Extremozymes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 161–176.
- Borje Lindstrom, E.; Wold, S.; Kettaneh-Wold, N. Optimization of Pyrite Bioleaching Using Sulfolobus acidocaldarius. Appl. Microbiol. Biotechnol. 1993, 38, 702–707.
- Brierley, C.L.; Brierley, J.A. Anaerobic Reduction of Molybdenum by Sulfolobus Species. Zent. Bakteriol. Mikrobiol. Hyg. I. Abt. Orig. C Allg. Angew. Okol. Mikrobiol. 1982, 3, 289–294.
- Rouchalova, D.; Rouchalova, K.; Janakova, I.; Cablik, V.; Janstova, S. Bioleaching of Iron, Copper, Lead, and Zinc from the Sludge Mining Sediment at Different Particle Sizes, PH, and Pulp Density Using Acidithiobacillus ferrooxidans. Minerals 2020, 10, 1013.
- Acevedo, F.; Gentina, J. Fundamentos y Perspectivas de las Tecnologías Biomineras, 1st ed.; Acevedo, F., Gentina, J., Eds.; Ediciones Universitarias de Valparaíso: Valparaiso, Chile, 2005.
- Rossi, G. Biohydrometallurgy, 1st ed.; McGraw-Hill: Hamburg, Germany, 1990.
- Rodríguez, Y. Contribución al Estudio Del Mecanismo de Biolixiviación de Distintos Sulfuros Metálicos Con Bacterias Mesófilas y Termófilas. Ph.D. Thesis, Universidad Complutense, Madrid, Spain, 2000.
- Berry, V.; Murr, L. Metallurgical Applications of Bacterial Leaching and Related Microbiological Phenomena, 1st ed.; Murr, L., Torma, A., Brierley, J., Eds.; Elsevier: New York, NY, USA, 1978; ISBN 9780125111508.
- Bennett, J.C.; Tributsch, H. Bacterial Leaching Patterns on Pyrite Crystal Surfaces. J. Bacteriol. 1978, 134, 310–317.
- Hansford, G.S.; Drossou, M. A Propagating Pore Model for the Batch Bioleach Kinetics of Refractory Gold-Bearing Pyrite. In Proceedings of the Biohydrometallurgy International Symposium, Warwick, UK, 12–16 July 1987; Norris, P.R., Kelly, D.P., Eds.; pp. 345–358.
- Tributsch, H. The Oxidative Desintegration of Sulfide Crystals by Thiobacillus ferrooxidans. Naturwissenschaften 1976, 63, 88.
- Newman, D.K. How Bacteria Respire Minerals. Science 2001, 292, 1312–1313.
- Sand, W.; Gehrke, T.; Jozsa, P.-G.; Schippers, A. (Bio)Chemistry of Bacterial Leaching—Direct vs. Indirect Bioleaching. Hydrometallurgy 2001, 59, 159–175.
- Masloboev, V.; Seleznev, S.; Svetlov, A.; Makarov, D. Hydrometallurgical Processing of Low-Grade Sulfide Ore and Mine Waste in the Arctic Regions: Perspectives and Challenges. Minerals 2018, 8, 436.
- Kim, B.-J.; Koh, Y.-K.; Kwon, J.-S. Bioleaching of Pyrrhotite with Bacterial Adaptation and Biological Oxidation for Iron Recovery. Metals 2021, 11, 295.
- Kovaříková, H.; Janáková, I.; Čablík, V.; Vrlíková, V. Bacterial Leaching of Polymetallic Ores from Zlatý Chlum Locality. J. Pol. Miner. Eng. Soc. 2019, 1, 145–158.
- Zhappar, N.K.; Shaikhutdinov, V.M.; Kanafin, Y.N.; Ten, O.A.; Balpanov, D.S.; Korolkov, I.V.; Collinson, S.R.; Erkasov, R.S.; Bakibaev, A.A. Bacterial and Chemical Leaching of Copper-Containing Ores with the Possibility of Subsequent Recovery of Trace Silver. Chem. Pap. 2019, 73, 1357–1367.
- Jin, Z.; Huang, T.; Zhang, X.; Zhang, S. Bioelectrochemical-Assisted Bioleaching of Chalcopyrite: Effect of Pulp Density, Anode Material, and Sliver Ion. Process Saf. Environ. Prot. 2022, 159, 740–748.
- Chen, S.-Y.; Wu, J.-Q.; Sung, S. Effects of Sulfur Dosage on Continuous Bioleaching of Heavy Metals from Contaminated Sediment. J. Hazard. Mater. 2022, 424, 127257.
- Yaashikaa, P.R.; Priyanka, B.; Senthil Kumar, P.; Karishma, S.; Jeevanantham, S.; Indraganti, S. A Review on Recent Advancements in Recovery of Valuable and Toxic Metals from E-Waste Using Bioleaching Approach. Chemosphere 2022, 287, 132230.
- Diaz, M.A.; De Ranson, I.U.; Dorta, B.; Banat, I.M.; Blazquez, M.L.; Gonzalez, F.; Muñoz, J.A.; Ballester, A. Metal Removal from Contaminated Soils Through Bioleaching with Oxidizing Bacteria and Rhamnolipid Biosurfactants. Soil Sediment Contam. Int. J. 2015, 24, 16–29.
- Yang, Z.; Zhang, Z.; Chai, L.; Wang, Y.; Liu, Y.; Xiao, R. Bioleaching Remediation of Heavy Metal-Contaminated Soils Using Burkholderia sp. Z-90. J. Hazard. Mater. 2016, 301, 145–152.
- Tran, T.M.; Han, H.-J.; Ko, J.-I.; Lee, J.-U. Effect of Indigenous Microbial Consortium on Bioleaching of Arsenic from Contaminated Soil by Shewanella Putrefaciens. Sustainability 2020, 12, 3286.
- Praburaman, L.; Park, J.-H.; Govarthanan, M.; Selvankumar, T.; Oh, S.-G.; Jang, J.-S.; Cho, M.; Kamala-Kannan, S.; Oh, B.-T. Impact of an Organic Formulation (Panchakavya) on the Bioleaching of Copper and Lead in Contaminated Mine Soil. Chemosphere 2015, 138, 127–132.
- Xu, M.; Liu, Y.; Deng, Y.; Zhang, S.; Hao, X.; Zhu, P.; Zhou, J.; Yin, H.; Liang, Y.; Liu, H.; et al. Bioremediation of Cadmium-Contaminated Paddy Soil Using an Autotrophic and Heterotrophic Mixture. RSC Adv. 2020, 10, 26090–26101.
- Hao, X.; Zhu, P.; Zhang, H.; Liang, Y.; Yin, H.; Liu, X.; Bai, L.; Liu, H.; Jiang, H. Mixotrophic Acidophiles Increase Cadmium Soluble Fraction and Phytoextraction Efficiency from Cadmium Contaminated Soils. Sci. Total Environ. 2019, 655, 347–355.
- Wu, C.; Jiang, M.; Hsieh, L.; Cai, Y.; Shen, Y.; Wang, H.; Lin, Q.; Shen, C.; Hu, B.; Lou, L. Feasibility of Bioleaching of Heavy Metals from Sediment with Indigenous Bacteria Using Agricultural Sulfur Soil Conditioners. Sci. Total Environ. 2020, 703, 134812.
- Gan, M.; Jie, S.; Li, M.; Zhu, J.; Liu, X. Bioleaching of Multiple Metals from Contaminated Sediment by Moderate Thermophiles. Mar. Pollut. Bull. 2015, 97, 47–55.
- Qayyum, S.; Meng, K.; Pervez, S.; Nawaz, F.; Peng, C. Optimization of PH, Temperature and Carbon Source for Bioleaching of Heavy Metals by Aspergillus Flavus Isolated from Contaminated Soil. Main Group Met. Chem. 2019, 42, 1–7.
- Porzionato, N.; Tufo, A.; Candal, R.; Curutchet, G. Metal Bioleaching from Anaerobic Sediments from Reconquista River Basin (Argentina) as a Potential Remediation Strategy. Environ. Sci. Pollut. Res. 2017, 24, 25561–25570.
- Gan, M.; Song, Z.; Zhu, J.; Liu, X. Efficient Bioleaching of Heavy Metals from Contaminated Sediment in Batch Method Coupled with the Assistance of Heterotrophic Microorganisms. Environ. Earth Sci. 2016, 75, 457.
- Nguyen, V.K.; Lee, J.-U. A Comparison of Microbial Leaching and Chemical Leaching of Arsenic and Heavy Metals from Mine Tailings. Biotechnol. Bioprocess Eng. 2015, 20, 91–99.
- Ye, M.; Li, G.; Yan, P.; Ren, J.; Zheng, L.; Han, D.; Sun, S.; Huang, S.; Zhong, Y. Removal of Metals from Lead-Zinc Mine Tailings Using Bioleaching and Followed by Sulfide Precipitation. Chemosphere 2017, 185, 1189–1196.
- Ye, M.; Yan, P.; Sun, S.; Han, D.; Xiao, X.; Zheng, L.; Huang, S.; Chen, Y.; Zhuang, S. Bioleaching Combined Brine Leaching of Heavy Metals from Lead-Zinc Mine Tailings: Transformations during the Leaching Process. Chemosphere 2017, 168, 1115–1125.
- Nguyen, V.K.; Lee, J.-U. Effect of Sulfur Concentration on Microbial Removal of Arsenic and Heavy Metals from Mine Tailings Using Mixed Culture of Acidithiobacillus spp. J. Geochem. Explor. 2015, 148, 241–248.
- Lee, E.; Han, Y.; Park, J.; Hong, J.; Silva, R.A.; Kim, S.; Kim, H. Bioleaching of Arsenic from Highly Contaminated Mine Tailings Using Acidithiobacillus thiooxidans. J. Environ. Manag. 2015, 147, 124–131.
- Falagán, C.; Grail, B.M.; Johnson, D.B. New Approaches for Extracting and Recovering Metals from Mine Tailings. Miner. Eng. 2017, 106, 71–78.
- Ahmadi, A.; Khezri, M.; Abdollahzadeh, A.A.; Askari, M. Bioleaching of Copper, Nickel and Cobalt from the Low Grade Sulfidic Tailing of Golgohar Iron Mine, Iran. Hydrometallurgy 2015, 154, 1–8.
- Ngoma, E.; Borja, D.; Smart, M.; Shaik, K.; Kim, H.; Petersen, J.; Harrison, S.T.L. Bioleaching of Arsenopyrite from Janggun Mine Tailings (South Korea) Using an Adapted Mixed Mesophilic Culture. Hydrometallurgy 2018, 181, 21–28.
- Martin, M.; Janneck, E.; Kermer, R.; Patzig, A.; Reichel, S. Recovery of Indium from Sphalerite Ore and Flotation Tailings by Bioleaching and Subsequent Precipitation Processes. Miner. Eng. 2015, 75, 94–99.
- Zhang, R.; Hedrich, S.; Römer, F.; Goldmann, D.; Schippers, A. Bioleaching of Cobalt from Cu/Co-Rich Sulfidic Mine Tailings from the Polymetallic Rammelsberg Mine, Germany. Hydrometallurgy 2020, 197, 105443.
- Mäkinen, J.; Salo, M.; Khoshkhoo, M.; Sundkvist, J.-E.; Kinnunen, P. Bioleaching of Cobalt from Sulfide Mining Tailings; a Mini-Pilot Study. Hydrometallurgy 2020, 196, 105418.
- Hao, X.; Liu, X.; Yang, Q.; Liu, H.; Yin, H.; Qiu, G.; Liang, Y. Comparative Study on Bioleaching of Two Different Types of Low-Grade Copper Tailings by Mixed Moderate Thermophiles. Trans. Nonferrous Met. Soc. China 2018, 28, 1847–1853.
- Huerta-Rosas, B.; Cano-Rodríguez, I.; Gamiño-Arroyo, Z.; Gómez-Castro, F.I.; Carrillo-Pedroza, F.R.; Romo-Rodríguez, P.; Gutiérrez-Corona, J.F. Aerobic Processes for Bioleaching Manganese and Silver Using Microorganisms Indigenous to Mine Tailings. World J. Microbiol. Biotechnol. 2020, 36, 124.
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of Lithium and Cobalt from Spent Lithium Ion Batteries (LIBs) Using Organic Acids as Leaching Reagents: A Review. Resour. Conserv. Recycl. 2018, 136, 418–435.
- de Wet, M.M.M.; Brink, H.G. Fungi in the Bioremediation of Toxic Effluents. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 407–431.
- Gadd, G.M. Microbial Influence on Metal Mobility and Application for Bioremediation. Geoderma 2004, 122, 109–119.
- Almudena, A.; Lizaso, J. Hongos y Micotoxinas; Fundación Ibérica para la Seguridad Alimentaria: Madrid, Spain, 2001.
- Serrano-Coll, H.; Cardona-Castro, N. Mycotoxicosis and Mycotoxins: Generalities and Basic Aspects. Rev. CES Med. 2015, 29, 143–151.
- Ismaiel, A.; Papenbrock, J. Mycotoxins: Producing Fungi and Mechanisms of Phytotoxicity. Agriculture 2015, 5, 492–537.
- Grewal, H.S.; Kalra, K.L. Fungal Production of Citric Acid. Biotechnol. Adv. 1995, 13, 209–234.
- Gadd, G.M. Fungal Production of Citric and Oxalic Acid: Importance in Metal Speciation, Physiology and Biogeochemical Processes. Adv. Microb. Physiol. 1999, 41, 47–92.
- Khan, I.; Qayyum, S.; Maqbool, F.; Mujaddad-ur-Rehman; Hayat, A.; Farooqui, M.S. Microbial Organic Acids Production, Biosynthetic Mechanism and Applications -Mini Review. Indian J. Geomarine. Sci. 2017, 46, 2165–2174.
- Roukas, T. Citric and Gluconic Acid Production from Fig by Aspergillus Niger Using Solid-State Fermentation. J. Ind. Microbiol. Biotechnol. 2000, 25, 298–304.
- Papagianni, M. Advances in Citric Acid Fermentation by Aspergillus Niger: Biochemical Aspects, Membrane Transport and Modeling. Biotechnol. Adv. 2007, 25, 244–263.
- Alongi, K.S.; Shields, G.C. Theoretical Calculations of Acid Dissociation Constants: A Review Article. Annu. Rep. Comput. Chem. 2010, 6, 113–138.
- Kütt, A.; Selberg, S.; Kaljurand, I.; Tshepelevitsh, S.; Heering, A.; Darnell, A.; Kaupmees, K.; Piirsalu, M.; Leito, I. PKa Values in Organic Chemistry–Making Maximum Use of the Available Data. Tetrahedron. Lett. 2018, 59, 3738–3748.
- Liu, M.; Ma, W.; Zhang, X.; Liang, Z.; Zhao, Q. Recycling Lithium and Cobalt from LIBs Using Microwave-Assisted Deep Eutectic Solvent Leaching Technology at Low-Temperature. Mater. Chem. Phys. 2022, 289, 126466.
- Wiecka, Z.; Rzelewska-Piekut, M.; Regel-Rosocka, M. Recovery of Platinum Group Metals from Spent Automotive Converters by Leaching with Organic and Inorganic Acids and Extraction with Quaternary Phosphonium Salts. Sep. Purif. Technol. 2022, 280, 119933.
- Verma, A.; Kore, R.; Corbin, D.R.; Shiflett, M.B. Metal Recovery Using Oxalate Chemistry: A Technical Review. Ind. Eng. Chem. Res. 2019, 58, 15381–15393.
- Martin, D.S.; Cole, R.J.; Haq, S. Investigating the Adsorption of Oxalic Acid onto Cu(110) to Create a Chemically Functionalised Surface. Surf. Sci. 2003, 539, 171–181.
- Sajadi, S.A.A. Metal Ion-Binding Properties of L-Glutamic Acid and L-Aspartic Acid, a Comparative Investigation. Nat. Sci. 2010, 2, 85–90.
- Deng, X.; Chai, L.; Yang, Z.; Tang, C.; Wang, Y.; Shi, Y. Bioleaching Mechanism of Heavy Metals in the Mixture of Contaminated Soil and Slag by Using Indigenous Penicillium Chrysogenum Strain F1. J. Hazard. Mater. 2013, 248–249, 107–114.
- Ramírez-Carmona, M.; Rendon-Castrillon, L.; Ocampo-López, C.; Giraldo-Aristizabal, R. Proceso Para La Separación de Metales En Una Matriz Sólida Mediante Lixiviación, Que Emplea Una Composición Que Contiene Ácidos Carboxílicos, Monosacáridos, Disacáridos, Aminoácidos, Ácidos Grasos, Alcoholes y Compuestos Fenólicos. Patent Number NC2019/0013648, 2022. 13.
- Pathak, A.; Vinoba, M.; Kothari, R. Emerging Role of Organic Acids in Leaching of Valuable Metals from Refinery-Spent Hydroprocessing Catalysts, and Potential Techno-Economic Challenges: A Review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1–43.
- Pathak, A.; Kothari, R.; Vinoba, M.; Habibi, N.; Tyagi, V.V. Fungal Bioleaching of Metals from Refinery Spent Catalysts: A Critical Review of Current Research, Challenges, and Future Directions. J. Environ. Manag. 2021, 280, 111789.
- Xia, M.; Bao, P.; Liu, A.; Wang, M.; Shen, L.; Yu, R.; Liu, Y.; Chen, M.; Li, J.; Wu, X.; et al. Bioleaching of Low-Grade Waste Printed Circuit Boards by Mixed Fungal Culture and Its Community Structure Analysis. Resour. Conserv. Recycl. 2018, 136, 267–275.
- Faraji, F.; Golmohammadzadeh, R.; Rashchi, F.; Alimardani, N. Fungal Bioleaching of WPCBs Using Aspergillus Niger: Observation, Optimization and Kinetics. J. Environ. Manag. 2018, 217, 775–787.
- Nili, S.; Arshadi, M.; Yaghmaei, S. Fungal Bioleaching of E-Waste Utilizing Molasses as the Carbon Source in a Bubble Column Bioreactor. J. Environ. Manag. 2022, 307, 114524.
- Chaerun, S.K.; Sulistyo, R.S.; Minwal, W.P.; Mubarok, M.Z. Indirect Bioleaching of Low-Grade Nickel Limonite and Saprolite Ores Using Fungal Metabolic Organic Acids Generated by Aspergillus Niger. Hydrometallurgy 2017, 174, 29–37.
- Rasoulnia, P.; Mousavi, S.M.; Rastegar, S.O.; Azargoshasb, H. Fungal Leaching of Valuable Metals from a Power Plant Residual Ash Using Penicillium Simplicissimum: Evaluation of Thermal Pretreatment and Different Bioleaching Methods. Waste Manag. 2016, 52, 309–317.
- Biswas, S.; Bhattacharjee, K. Fungal Assisted Bioleaching Process Optimization and Kinetics: Scenario for Ni and Co Recovery from a Lateritic Chromite Overburden. Sep. Purif. Technol. 2014, 135, 100–109.
- Wu, H.-Y.; Ting, Y.-P. Metal Extraction from Municipal Solid Waste (MSW) Incinerator Fly Ash—Chemical Leaching and Fungal Bioleaching. Enzym. Microb. Technol. 2006, 38, 839–847.
- Alavi, N.; Partovi, K.; Majlessi, M.; Rashidi, M.; Alimohammadi, M. Bioleaching of Metals from Cellphones Batteries by a Co-Fungus Medium in Presence of Carbon Materials. Bioresour. Technol. Rep. 2021, 15, 100768.
- Shah, S.S.; Palmieri, M.C.; Sponchiado, S.R.P.; Bevilaqua, D. Enhanced Bio-Recovery of Aluminum from Low-Grade Bauxite Using Adapted Fungal Strains. Braz. J. Microbiol. 2020, 51, 1909–1918.
- Moazzam, P.; Boroumand, Y.; Rabiei, P.; Baghbaderani, S.S.; Mokarian, P.; Mohagheghian, F.; Mohammed, L.J.; Razmjou, A. Lithium Bioleaching: An Emerging Approach for the Recovery of Li from Spent Lithium Ion Batteries. Chemosphere 2021, 277, 130196.
- Khan, I.; Aftab, M.; Shakir, S.; Ali, M.; Qayyum, S.; Rehman, M.U.; Haleem, K.S.; Touseef, I. Mycoremediation of Heavy Metal (Cd and Cr)–Polluted Soil through Indigenous Metallotolerant Fungal Isolates. Environ. Monit. Assess. 2019, 191, 585.
- Sierra-Alvarez, R. Removal of Copper, Chromium and Arsenic from Preservative-Treated Wood by Chemical Extraction-Fungal Bioleaching. Waste Manag. 2009, 29, 1885–1891.
- Qu, M.; Chen, J.; Huang, Q.; Chen, J.; Xu, Y.; Luo, J.; Wang, K.; Gao, W.; Zheng, Y. Bioremediation of Hexavalent Chromium Contaminated Soil by a Bioleaching System with Weak Magnetic Fields. Int. Biodeterior. Biodegrad. 2018, 128, 41–47.
- Zeng, X.; Wei, S.; Sun, L.; Jacques, D.A.; Tang, J.; Lian, M.; Ji, Z.; Wang, J.; Zhu, J.; Xu, Z. Bioleaching of Heavy Metals from Contaminated Sediments by the Aspergillus Niger Strain SY1. J. Soils Sediments 2015, 15, 1029–1038.
- Seh-Bardan, B.J.; Othman, R.; Wahid, S.A.; Husin, A.; Sadegh-Zadeh, F. Bioleaching of Heavy Metals from Mine Tailings by Aspergillus Fumigatus. Bioremediat. J. 2012, 16, 57–65.
- Magnuson, J.K.; Lasure, L.L. Organic Acid Production by Filamentous Fungi. In Advances in Fungal Biotechnology for Industry, Agriculture, and Medicine; Springer: Boston, MA, USA, 2004; pp. 307–340.
- Shankar, T.; Sivakumar, T. Optimization of Citric Acid Production Using Aspergillus Niger Isolated from the Leaf Litter Soil of Sathuragiri Hills. Univers. J. Microbiol. Res. 2016, 4, 79–87.
- Oraby, E.A.; Eksteen, J.J. The Selective Leaching of Copper from a Gold–Copper Concentrate in Glycine Solutions. Hydrometallurgy 2014, 150, 14–19.
- Niu, Z.; Zou, Y.; Xin, B.; Chen, S.; Liu, C.; Li, Y. Process Controls for Improving Bioleaching Performance of Both Li and Co from Spent Lithium Ion Batteries at High Pulp Density and Its Thermodynamics and Kinetics Exploration. Chemosphere 2014, 109, 92–98.
This entry is offline, you can click here to edit this entry!
