Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cellulose nanocrystals (CNCs) are emerging nanomaterials derived from the most abundant renewable polymer on earth, being widely distributed in plants, bacteria, algae, etc., which can be extracted from these cellulosic sources through mechanical disintegration, controlled sulfuric acid hydrolysis and mixed acid hydrolysis.
- cellulose nanocrystals
- preparation methods
- rheological properties
1. Introduction
Cellulose nanocrystals (CNCs) are emerging nanomaterials derived from the most abundant renewable polymer on earth, being widely distributed in plants, bacteria, algae, etc., which can be extracted from these cellulosic sources through mechanical disintegration, controlled sulfuric acid hydrolysis and mixed acid hydrolysis [1][2][3][4][5]. In particular, sulfuric acid hydrolysis is among the most commonly used methods, whereby the glycosidic bonds are hydrolyzed and, simultaneously, a fraction of the surface hydroxyl groups is esterified to sulfate half-ester groups (Figure 1) [3][6][7]. However, it is important to note that there are other acids employed for acid hydrolysis, including hydrochloric acid, nitric acid and phosphoric acid [8][9][10]. Additionally, alternative methods for obtaining CNCs exist, such as mechanical treatments [11][12], and enzymatic and ionic liquid hydrolysis [13]. For a more detailed description of CNC fabrication, the reference [14] is recommended to the reader.
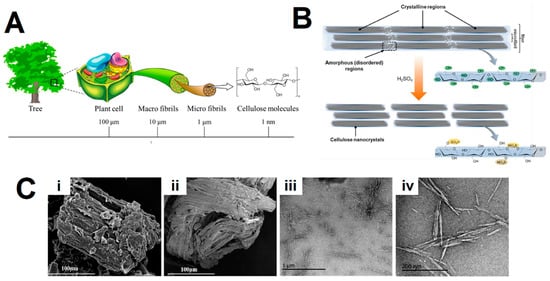
Figure 1. (A) Cellulose contained in plants or trees has a hierarchical structure from the meter to the nanometer scale. Adapted from reference [5]. (B) Schematics of idealized cellulose fibers showing one of the suggested configurations of the crystalline and amorphous regions, and CNCs after sulfuric acid hydrolysis of the amorphous regions, exhibiting the characteristic sulfate half-ester surface groups formed as a side reaction. Adapted from reference [6]. (C) Scanning electron microscope (SEM) images of (i) the raw corn stalk, (ii) the extracted cellulose and (iii,iv) transmission electron microscopy (TEM) images of the isolated cellulose nanocrystals (CNCs) using sulfuric acid hydrolysis. Adapted from reference [7].
This results in partly to fully crystalline regions, consisting of packed cellulose chains approximately 280 β(1−4) linked D-glucose units long, which have attracted great attention across several applications, including biomedical devices, food modifiers and cosmetic materials [15]. In fact, CNCs provide a promising alternative to inorganic fillers and stand out in terms of their material properties. In this sense, it has been used by several research groups because they display high strength (elastic modulus, 110–220 GPa; tensile strength, 7.5–7.7 GPa), low density (1.6 g/cm3), colloidal stability, nanoscale dimensions, high crystallinity, good biocompatibility, high surface area and tunable surface chemistry, enabling interactions with nanoparticles, small molecules, polymers and biological materials [16][17]. The high surface-area-to-volume ratio associated with the nanoscale dimensions can greatly affect the properties of other materials when added as composite, providing several sites for chemical reactions, as well as the adsorption of molecules, such as drugs. Importantly, the high aspect ratio allows the CNCs to self-assemble into the liquid crystalline phase, which can result in ordered structures that stabilize interfaces and form percolated networks [18][19].
Following these lines, CNCs have garnered considerable interest as composites in a wide variety of hydrophilic and hydrophobic composite matrices, such as rheological modifiers in industrial fluids, cosmetics, food, paints, lubricants and household formulated products [3][20], but they have also found several applications as optical, electroconductive and biomedical materials [21]. However, the charged interface of CNCs and their tendency to aggregate in more hydrophobic solvents complicates the efficient dispersion in most conventional polymeric materials, despite the fact that this can be overcome with surface functionalization [22]. Conversely, CNCs have been regarded as suitable and promising materials for the development of hydrogels, owing to their rigidity, mechanical strength, hydrophilicity, global abundance and biocompatibility [23]. In general, hydrogels, which are highly hydrated cross-linked three-dimensional networks, can be prepared by either using chemical or physical cross-linking techniques and encompass a wide range of chemical compositions and structural forms [23][24]. CNC-based gels are commonly prepared through the addition of CNCs to a polymer precursor solution, followed by chemical or physical cross-linking methods to induce the gelation. In some cases, the chemical cross-linking methods require surface modification of CNCs to create cross-linking sites [22] and the use of chemical agents, such as glutaraldehyde or epichlorohydrin, to form covalent bonds between the CNCs and polymer network, whereas physical cross-linking methods involve the use of heat, pressure or shear forces to form gels. Recently, pristine and modified cellulose nanocrystals have been introduced into various synthetic polymer matrices, such as poly(oligo) ethylene glycol methacrylate (POEGMA), poly(acrylic acid) (PAA), poly(acrylamide) (PAM), polyvinyl alcohol (PVA), poly(ethylene glycol) (PEG), and natural polymers (e.g., gelatin, alginate) as reinforcing agents for the preparation of more mechanical stable hydrogels [21][25], which have been of interest for biomedical applications [22][26]. Whereas the rigidity and mechanical strength endow CNCs as suitable reinforcing agents (or fillers) of polymeric hydrogel networks, the CNCs are ill-suited as single-component gels due to the lack of their ability to entangle with each other. Yet, the destabilization of CNC suspensions through modification of the surface chemistry, such as by salt or acid addition, polymer grafting, cross-linking with multivalent ions or the introduction of chemical bonds, can reduce the repulsion between particles and promote gel formation [25][27][28][29].
The outstanding mechanical stability, shear-thinning behavior, tunable mechanical properties, high water-holding capacity, controllable morphology, high biocompatibility and biodegradability contribute to the wide interest in using CNC-based gels for applications in various fields such as cosmetics, drug delivery, tissue engineering and the food industry [22][30][31][32]. For instance, in the cosmetic industry, CNC gels can be used as thickeners, emulsifiers and stabilizers in formulations. The high biocompatibility and suitable mechanical properties further enable the CNC gels as matrices for controlled drug release, as scaffolds to support the growth of cells and tissues, and as materials to improve the texture and stability of food products.
2. Preparation and Properties of CNC Gels
2.1. Preparation Methods
2.1.1. Preparation of CNC-Based Hydrogels
As mentioned, CNCs lack the ability to entangle with each other and form stable hydrogels, owing to the rigid structure. Yet, few reports have presented the development of CNC-only gels either by surface functionalization [28] or through cross-linking with multivalent ions or chemical bonds [27]. In general, the CNC critical gelation concentration and gel properties can be tuned by varying the solution conditions (e.g., ionic strength and pH), surface functionalization, addition of (non-)adsorbing water polymers and/or surfactants and temperature [27][28][33][34][35]. Increasing the CNC concentration leads to a decrease in the electrostatic double layer distance between CNCs, and consequently promoting gelation [36][37][38]. In addition, increasing the ionic strength leads to the screen of surface charge, which consequently can lead to gelation as it suppresses the electrostatic repulsion and enables dominant attractive forces (e.g., van der Waals and hydrogen bonding) between CNCs. Chau et al. [27] demonstrated that the charge number and ionic radii led to an increase in gel stiffness, but had little impact on the critical gelation concentration. The functionalization of CNC with carboxyl or amine groups, to provide pH-responsive gelation (Figure 2A), was demonstrated by Way et al. [28]. The treatment of cellulose with 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) can endow the CNC surface with carboxyl groups [39]. Carboxylic acid-modified CNCs can be protonated at a lower pH, which allows the domination of the attractive forces, while at a high pH the repulsive forces inhibit the aggregation [28]. The opposite behavior is observed for the amine-functionalized CNCs, which aggregate at a high pH. Furthermore, the authors demonstrated that the pH-responsive CNCs could be incorporated into a poly(vinyl acetate) matrix to produce mechanically adaptive pH-responsive nanocomposite films. Lewis et al. [35] employed a hydrothermal treatment of CNC suspensions to obtain gels, which was associated with the desulfation at high temperatures. In addition, the viscosity and storage modulus were found to be proportional to the treatment temperature. CNC desulfation was also demonstrated by Dorris et al. [40] to be a requirement to induce gelation in dilute CNC suspensions in a glycerol/water mixture. Recently, 3D printing has been recognized as a promising technology to fabricate in vitro model and constructs for tissue engineering and regenerative medicine [41]. Among the several types of hydrogels, the CNCs have a huge potential owing to the biocompatibility, good mechanical properties and anisotropic shape that can be aligned under shear-induced conditions to control the structure and function of gels. For instance, Ma et al. [29] demonstrated that the shear-induced self-assembly of CNCs at 20 wt% during 3D printing could provide gels with optimal print resolution and fidelity (Figure 2B).
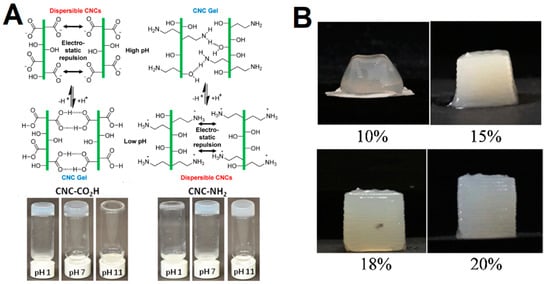
Figure 2. (A) Schematic representation of the proposed interactions between CNC-CO2H and CNC-NH2 at high and low pH; Images of aqueous dispersions of 2.7 wt% CNC-CO2H and 2.7 wt% CNC-NH2 at pH 1, 7, and 11. Adapted from reference [28]. (B) Visual appearance of 1 cm3 cubes printed with CNC hydrogels of increasing solid loading. Adapted from reference [29].
2.1.2. Hydrogels with Physically Cross-Linked CNCs
The CNCs’ properties make these materials suitable as reinforcing agents, which are incorporated in polymeric matrices, either through physical or chemical methods. Up to now, the majority of the reported CNC hydrogels have mostly explored the physical incorporation of CNCs as reinforcing agents (or fillers) into polymeric hydrogel networks, in which the cross-linking between CNCs and polymer networks is achieved through physical interaction (such as electrostatic interaction, hydrophobic interaction, hydrogen bonding, and van der Waals forces). Common methods include homogenization and physical cross-linking of the polymer network [42][43][44][45][46][47][48], cyclic freeze–thaw processing [49][50], and chemical cross-linking of the polymeric species within the CNCs’ dispersion, such as free radical polymerization [51][52][53][54][55] and UV mediated cross-linking [56][57][58]. These methods have been widely employed with several network polymers including poly(vinyl alcohol) (PVA), polyacrylamides (PAM) and poly(ethylene glycol) (PEG)-based materials, as well as natural polymers (e.g., agarose, alginate and gelatin), which could form more mechanically stable hydrogels. Among the mentioned methods, the homogenization method provides a simple means to obtain reinforced gels, in which the gelation is triggered by mixing a CNC dispersion with a polymer [59]. The strong hydrogen bonds between polymers and CNCs have also been explored with synthetic polymers such as poly(N-isopropylacrylamide) (PNIPAM). Chen et al. [60] demonstrated that the incorporation with CNCs in these gels reinforces the mechanical properties and allow gels to extend more than 20 times their initial length. Another simple method includes the use of thermoresponsive hydrogels that attain the gel state upon a temperature variation. For instance, You et al. [16] developed in situ gelling hydrogels based on quaternized cellulose (QC) and CNCs that could immediately form gels upon an increase in temperature. The physical cross-linking can also be achieved through ionic/electrostatic interaction, i.e., cross-linking by ionic bonds, which are advantageous due to the mild reaction conditions, good performance at room temperature and not requiring organic solvents [61]. You et al. [62] developed a biocompatible and pH-responsive hydrogel consisting of two functional polymeric chains, which consisted of 5-aminolevulinic acid and dopamine conjugated to CNC through the coordination of iron, ALA/Fe@CNC and PDA/Fe@CNC, respectively. The authors further demonstrated that both chains displayed different functions, with the PDA/Fe@CNC chain enabling an increased cell adhesion, while the ALA/Fe@CNC chain enhanced reactive oxygen species (ROS) production. In addition, the iron coordination endowed the system with pH-responsiveness, which was explored for the pH-triggered release of paclitaxel. Host–guest interactions have also been a useful strategy to achieve gels with good mechanical properties, which depend on making use of the non-covalent binding between molecules due to their unique structures. In particular, cyclodextrins are widely used in supramolecular hydrogels to obtain host–guest interactions, owing to the self-assembly with polymeric chains [63][64]. Moreover, the gels obtained through this means usually display thixotropism, making them suitable for syringeable drug delivery application [65]. Lin et al. [66] grafted CNCs with β-cyclodextrin (CD), and Pluronic polymers (poloxamer) were introduced on the surface of CNC, owing to the interaction between CD and hydrophobic polypropylene glycol segments of the polymer, forming stable complexes after ultrasound-assisted stirring. The authors demonstrated the control of the mechanical properties and temperature responsiveness by changing the CD/CNCs ratio. The freeze–thaw method has also been widely explored for production of a strong physical gel network. In this method, a phase separation occurs as the solution freezes and the polymer is rejected from the growing ice crystallites, which, upon melting of the ice (thaw), results in water-filled pores surrounded by a polymer skeleton [50][67][68]. The ice crystallites size increases with repeated freeze–thaw cycles, which, together with the solvent, pH, temperature and time, can be used to modulate several properties, such as the degree of gelation, stability and mechanical properties. Gonzalez et al. [49] developed CNC/PVA gels by a freezing–thawing technique, in which the CNCs could work as nucleation sites that led to improved mechanical properties and thermal stability, without affecting the transparency of the samples. As mentioned, gels can also be obtained from chemical cross-linking of the polymeric species within the CNCs’ dispersion [69][70][71]. De France et al. [69] obtained injectable gels by physically incorporating CNCs into hydrazone cross-linked poly(oligoethylene glycol methacrylate) (POEGMA) hydrogels through co-extrusion of the reactive precursor polymer solutions from a double-barrel syringe (Figure 3A). The authors observed that the incorporation of 5 wt% CNCs could strongly enhance mechanical properties (up to 35-fold increases in storage modulus), in addition to enabling faster gelation rates, decreased swelling ratios and increased stability. Finally, 3D printing has also been a recently employed method to improve the control over the gels’ properties, such as size, shape, pore structure and pore orientation [72][73]. Sultan et al. [73] fabricated double-cross-linked interpenetrating polymer network (IPN) hydrogels consisting of sodium alginate (SA) and gelatin (G) reinforced with cellulose nanocrystals (CNCs) (Figure 3B). Initially, CNCs were mixed with SA and G to form hydrogel ink at the ratio of 70/20/10 (wt%), which was printed with different structures and cross-linked sequentially via covalent and ionic reactions using CaCl2 and glutaraldehyde, respectively.
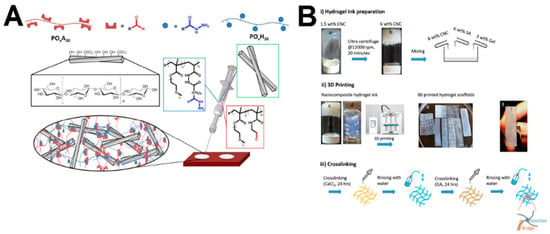
Figure 3. (A) Schematic representation of injectable CNC-reinforced poly(oligoethylene glycol methacrylate) (POEGMA) gels. Adapted from reference [69]. (B) Schematic representation of the processing route for 3D-printed nanocomposites hydrogel scaffolds of sodium alginate (SA) and gelatin (G) reinforced with cellulose nanocrystals (CNCs). Adapted from reference [73].
2.1.3. Hydrogels with Chemically Cross-Linked CNCs
The chemical cross-linking consists of the formation of covalent bonds between polymer chains and CNCs, which mandates the surface modification of CNCs with specific functional groups, such as silyl groups, carboxyl or aldehyde groups, to create cross-linking sites. Yet, some works have reported the cross-linking of unmodified CNCs through free radical polymerization using small molecule cross-linkers [74][75][76]. The modification can be achieved by direct surface chemical modification or through physical interaction/adsorption of molecules to the surface of the CNCs [77]. Compared to the physical gels, the loading of CNCs is similar but displays larger mechanical stability and typically a higher storage modulus, which can result from the new covalent bonds formed between the CNCs and the surrounding hydrogel network. Additionally, the chemical incorporation of CNCs might consist of either CNCs acting as cross-linkers themselves or the CNCs cross-linked to a network polymer matrix. Among the reported preparation methods, the most common have been the homogenization [78][79][80][81][82] and free radical polymerization [74][75][76][83][84][85][86][87], while other methods are less described, including UV polymerization [88], freeze-casting [89][90] and coextrusion [91][92]. The majority of the reported studies consist of polyacrylamide/polyacrylate-based hydrogels. For instance, Yan et al. [93] cross-linked silane-modified CNCs with polymer chains of poly(N,N-dimethylacrylamide) (PDMA) through free radical polymerization (Figure 4A). The mechanism consists of the nucleation of the silane-modified CNCs in an acrylamide monomer solution that leads to hierarchically structured CNC–PDMA clusters through physical interactions to form a percolated network. The authors observed that the hybrid gels displayed higher mechanical properties and a more efficient energy dissipation mechanism than the control PDMA hydrogels. Chau et al. [89] fabricated, through a freeze-casting-based fabrication method, hydrogels with reversible hydrazone cross-links between hydrazide-modified poly(oligoethylene glycol methacrylate) (POEGMA) and aldehyde-functionalized CNCs (Figure 4B). The authors reported that the composite hydrogels displayed high structural and mechanical integrity and a strong variation in Young’s moduli in orthogonal directions that could be explored as effective biomimetic scaffolds for oriented tissues. Moreover, the pore morphologies could be tuned by varying the concentration and CNC-to-POEGMA ratio, and displayed a directionally dependent swelling behavior. The aldehyde–hydrazide chemistry has been commonly explored to obtain injectable gels [91][92]. Nonetheless, other strategies have also been explored, such as the use of Diels-Alder “click” cross-linking [82], and Schiff-base linkage [25][93][94][95][96]. Tang et al. [25] developed hydrogels comprising CNCs and sodium alginate (SA) that were initially oxidized to impart aldehyde functional groups that served as reaction sites for amine-containing vinyl functionalized monomers. In this way, the CNCs worked as cross-linkers that ensured a good structural integrity and mechanical stability of the hydrogels, while the dynamic Schiff-base linkage endowed the gels with self-healing properties. Liu et al. [97] prepared an injectable polysaccharide hydrogel based on cellulose acetoacetate (CAA), hydroxypropyl chitosan (HPCS), and amino-modified cellulose nanocrystals (CNC-NH2) under physiological conditions. The CNC-NH2 were found to act both as a physical and chemical cross-linker, for which the concentration could affect the mechanical properties, internal morphology and gelation time. In addition, the authors reported that the hydrogel exhibited pH-responsive properties, excellent stability under physiological conditions, and self-healing behavior under acidic conditions, via enamine bond exchange.
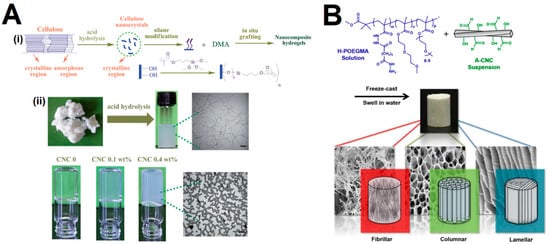
Figure 4. (A) (i) Schematic representation of the preparation of silane-modified CNCs to fabricate nanocomposite hydrogels, and (ii) TEM images (bar = 100 nm) of the CNCs after acid hydrolysis and the percolating gels obtained from free radical polymerization of poly(N,N-dimethylacrylamide) (DMA). Adapted from reference [93]. (B) Schematic representation of the fabrication of CNC-POEGMA nanocomposite hydrogels formed through hydrazone cross-linking of aldehyde-modified CNCs (A-CNCs) with hydrazide-functionalized POEGMA (H-POEGMA) and subsequent directional freeze casting, and the SEM images of the resulting hydrogels’ morphologies (fibrillar, columnar, lamellar). Adapted from reference [89].
2.2. Rheological Properties
The hydrogels are usually evaluated by dynamic mechanical properties, i.e., the elastic (or storage) modulus (G’) (also known as the dynamic rigidity), reflecting the reversibly stored energy of the system, and the viscous (loss) modulus (G’’), reflecting the irreversible energy loss. These studies are used to examine the stability change of material in the sol-gel transition process. The hydrogels’ dynamic mechanical properties are measured as a function of frequency by oscillatory rheological measurements and can be used for different geometries in a rheometer, like a plate and Couette geometry. When G’ is above G”, this means that the elasticity is dominant, which implies that the gelation process is prevailing. In contrast, G” above G’ represents a viscosity dominated solution-like material. When plotted against frequency, a pronounced plateau is present in the G’ modulus spectrum for rigid gel structures, whilst the G’’ modulus should be considerably smaller than G’ in the plateau region [98]. The loss factor (also known as the damping factor) is defined as the ratio of lost energy to storage energy during deformation (tan δ = G”/G’). Typically, the values tan δ < 1 correspond to a true gel’s plateau region [99].
In the studies about CNC hydrogel nanocomposites, the aim most found was to study the effect of different concentrations of CNCs on the elastic modulus (G’) and viscous modulus (G’’), and the influence on the gelation process. In general, the authors showed an increase in G’ and G’’ with an increase in the CNCs’ concentration, and the hydrogels exhibited a predominantly elastic response, with the elastic modulus G’ exceeding the viscous modulus G’’. Moreover, they showed that addition of CNCs can clearly control the gelation process of hydrogel solutions or suspensions [25][71][97][100].
Among the reported studies, some explored the effect of different concentrations of pure and modified CNCs (acting as a cross-linking agent) on rheological properties. For example, as mentioned, Tang et al. [25] incorporated oxidized CNCs (CNC-CHO) into oxidized sodium alginate (Alg-CHO), and the results showed a 2-, 5-, and 10-fold increase in the storage modulus for hydrogel reinforced with 0.5, 1.0, 1.5 wt% CNC-CHO, respectively. The authors also measured the complex viscosity (η*) that displayed a linear function of log ω with a slope of -1, indicating that the relaxation time was much greater than the experimental time. However, when the amount of oxidized CNCs was increased, the relaxation time of the network decreased. Liu et al. [97] employed amino-modified CNCs (CNC-NH2) to reinforce a polysaccharide mixture of cellulose acetoacetate (CAA) and hydroxyproyl chitosan (HPCS). They showed that the G’ of the CAA/HPCS hydrogel increased with the addition of different concentrations of pure CNCs and the amino-modified (CNCs-NH2). Importantly, besides that both particles induced an increase in G’ with an increase in the CNCs loading up until a critical concentration, the modulus always remained greater for CNC-NH2, as it could act both as a filler and cross-linker in the hydrogel. Beyond the critical concentration, the excess CNCs could form aggregates that lead to the phase separation of the suspension, which inhibits the cross-linking between HPCS and CAA, and consequently leads to a decrease in the G’ values. You et al. [62] demonstrated that simple entrapment of cationic CNCs (CCNCs) within cationic cellulose-based hydrogels formed in situ could result in injectable hydrogels with increasing orders-of-magnitude in the mechanical strength, with the increase in CCNCs’ concentration (nearly a 200-fold increase in G’ at 2.5 wt% of CCNCs compared to the neat hydrogels). The authors associated the improvement of the mechanical strength and dimensional stability of the hydrogels to the strong interaction between CCNCs and QC, mediated by the cross-linking agent (β-glycerophosphate). As mentioned above, the chemical incorporation of CNCs leads to a mechanical improvement that surpasses the physically incorporated counterparts. For example, Han et al. [42] observed that the use of borax as a cross-linker agent in a mixture of CNCs and PVA could improve the interaction between both components, which led to nearly an order of magnitude enhancement of the G’ and η* than in gels without borax, indicating an important role of borax in the 3D network structure. Ooi et al. [71] also reported an increase in G’ and G’’ in gelatin-based hydrogels with an increasing concentration of CNC, despite the fact that gels became more solid-like and did not display a critical concentration of CNCs. However, the effect of the cross-linking agent (glutaraldehyde) is not mentioned. Zhou et al. [74] employed CNCs to reinforce polyacrylamide (PAM) hydrogels obtained through in situ free-radical polymerization in the presence of the cross-linker N,N′-methylenebisacrylamide (NMBA). The authors reported that the CNCs acted not only as a reinforcing agent for hydrogels, but also as a multifunctional cross-linker for gelation, as it resulted in a faster gelation and increased effective cross-link density. Additionally, the good dispersion of CNCs in PAM and the enhanced interfacial interaction between both components led to a significant increase in the shear storage modulus, compression strength and elastic modulus of the nanocomposite hydrogel. Additionally, the molar mass of the polymers can also affect the properties of gels, as it is the case for gels obtained by mixing CNCs with polymers. For instance, Talankite et al. [59] evaluated the effect of xyloglucan (XG) molar mass on the formation and mechanical properties of XG/CNC hydrogels. The authors reported that in the case of low molar mass XG, the samples displayed the behavior of a viscoelastic liquid, in which G’ and G’’ were very low and almost superimposed. However, the increase in the XG molar mass resulted in samples with larger G’ and G’’ and a more solid-like behavior. Therefore, the gelation was associated with the formation of XG/CNC complexes, inducing an increase in the effective hydrodynamic volume of CNC and leading to the interaction and entanglement between XG loops and tails. As a higher molar mass resulted in a higher effective hydrodynamic volume of XG/CNCs, the gelation could occur at a lower XG/CNC ratio. Finally, it is also noticed that the CNCs’ size and aspect ratio can also affect the properties of CNC-based gels, as observed in the works of Han et al. [42] and Ling et al. [101]. In the former, the incorporation of cellulose nanofibers could induce a stronger enhancement of the mechanical properties than CNCs. In the latter, the authors evaluated the influence of cellulose nanoparticles with fibrous, spherical and rod-like form on the rheological properties of CNC and CNC-poly(vinyl alcohol) (PVA) suspensions [101]. The authors reported that the morphology played a major role in the rheology of the suspensions, in which a larger size (dimension and length) contributed to a higher viscosity value, a more complex state-flow rheological behavior and more solid-like viscoelastic behavior.
This entry is adapted from the peer-reviewed paper 10.3390/gels9070574
References
- Le Gars, M.; Douard, L.; Belgacem, N.; Bras, J. Cellulose Nanocrystals: From Classical Hydrolysis to the Use of Deep Eutectic Solvents. In Smart Nanosystems for Biomedicine, Optoelectronics and Catalysis; IntechOpen: Rijeka, Croatia, 2020.
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials. Int. J. Polym. Sci. 2018, 2018, 7923068.
- Vanderfleet, O.M.; Cranston, E.D. Production Routes to Tailor the Performance of Cellulose Nanocrystals. Nat. Rev. Mater. 2020, 6, 124–144.
- Capron, I.; Rojas, O.J.; Bordes, R. Behavior of Nanocelluloses at Interfaces. Curr. Opin. Colloid Interface Sci. 2017, 29, 83–95.
- Miyashiro, D.; Hamano, R.; Umemura, K. A Review of Applications Using Mixed Materials of Cellulose, Nanocellulose and Carbon Nanotubes. Nanomaterials 2020, 10, 186.
- Domingues, R.M.A.; Gomes, M.E.; Reis, R.L. The Potential of Cellulose Nanocrystals in Tissue Engineering Strategies. Biomacromolecules 2014, 15, 2327–2346.
- Huang, S.; Zhou, L.; Li, M.-C.; Wu, Q.; Zhou, D. Cellulose Nanocrystals (CNCs) from Corn Stalk: Activation Energy Analysis. Materials 2017, 10, 80.
- Cheng, M.; Qin, Z.; Chen, Y.; Hu, S.; Ren, Z.; Zhu, M. Efficient Extraction of Cellulose Nanocrystals through Hydrochloric Acid Hydrolysis Catalyzed by Inorganic Chlorides under Hydrothermal Conditions. ACS Sustain. Chem. Eng. 2017, 5, 4656–4664.
- Cheng, M.; Qin, Z.; Hu, J.; Liu, Q.; Wei, T.; Li, W.; Ling, Y.; Liu, B. Facile and Rapid One–Step Extraction of Carboxylated Cellulose Nanocrystals by H2SO4/HNO3 Mixed Acid Hydrolysis. Carbohydr. Polym. 2020, 231, 115701.
- Amin, K.N.M.; Hosseinmardi, A.; Martin, D.J.; Annamalai, P.K. A Mixed Acid Methodology to Produce Thermally Stable Cellulose Nanocrystal at High Yield Using Phosphoric Acid. J. Bioresour. Bioprod. 2022, 7, 99–108.
- Sofla, M.R.K.; Brown, R.J.; Tsuzuki, T.; Rainey, T.J. A Comparison of Cellulose Nanocrystals and Cellulose Nanofibres Extracted from Bagasse Using Acid and Ball Milling Methods. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035004.
- Amin, K.N.M.; Annamalai, P.K.; Morrow, I.C.; Martin, D. Production of Cellulose Nanocrystals via a Scalable Mechanical Method. RSC Adv. 2015, 5, 57133–57140.
- Tang, Y.; Yang, H.; Vignolini, S. Recent Progress in Production Methods for Cellulose Nanocrystals: Leading to More Sustainable Processes. Adv. Sustain. Syst. 2022, 6, 2100100.
- Hamad, W.Y. Cellulose Nanocrystals: Properties, Production and Applications; Wiley: Hoboken, NJ, USA, 2017; ISBN 978-1-119-96816-0.
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392.
- You, J.; Cao, J.; Zhao, Y.; Zhang, L.; Zhou, J.; Chen, Y. Improved Mechanical Properties and Sustained Release Behavior of Cationic Cellulose Nanocrystals Reinforeced Cationic Cellulose Injectable Hydrogels. Biomacromolecules 2016, 17, 2839–2848.
- Shojaeiarani, J.; Bajwa, D.S.; Chanda, S. Cellulose Nanocrystal Based Composites: A Review. Compos. Part C Open Access 2021, 5, 100164.
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current International Research into Cellulose Nanofibres and Nanocomposites. J. Mater. Sci. 2010, 45, 1–33.
- Revol, J.-F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal Self-Ordering of Cellulose Microfibrils in Aqueous Suspension. Int. J. Biol. Macromol. 1992, 14, 170–172.
- Nagarajan, K.J.; Ramanujam, N.R.; Sanjay, M.R.; Siengchin, S.; Surya Rajan, B.; Sathick Basha, K.; Madhu, P.; Raghav, G.R. A Comprehensive Review on Cellulose Nanocrystals and Cellulose Nanofibers: Pretreatment, Preparation, and Characterization. Polym. Compos. 2021, 42, 1588–1630.
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose Nanocrystals and Cellulose Nanofibrils Based Hydrogels for Biomedical Applications. Carbohydr. Polym. 2019, 209, 130–144.
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631.
- Shojaeiarani, J.; Bajwa, D.; Shirzadifar, A. A Review on Cellulose Nanocrystals as Promising Biocompounds for the Synthesis of Nanocomposite Hydrogels. Carbohydr. Polym. 2019, 216, 247–259.
- Kim, H.J.; Jeong, J.H.; Choi, Y.H.; Eom, Y. Review on Cellulose Nanocrystal-Reinforced Polymer Nanocomposites: Processing, Properties, and Rheology. Korea-Aust. Rheol. J. 2021, 33, 165–185.
- Tang, J.; Javaid, M.U.; Pan, C.; Yu, G.; Berry, R.M.; Tam, K.C. Self-Healing Stimuli-Responsive Cellulose Nanocrystal Hydrogels. Carbohydr. Polym. 2020, 229, 115486.
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a Tiny Fiber with Huge Applications. Curr. Opin. Biotechnol. 2016, 39, 76–88.
- Chau, M.; Sriskandha, S.E.; Pichugin, D.; Thérien-Aubin, H.; Nykypanchuk, D.; Chauve, G.; Méthot, M.; Bouchard, J.; Gang, O.; Kumacheva, E. Ion-Mediated Gelation of Aqueous Suspensions of Cellulose Nanocrystals. Biomacromolecules 2015, 16, 2455–2462.
- Way, A.E.; Hsu, L.; Shanmuganathan, K.; Weder, C.; Rowan, S.J. PH-Responsive Cellulose Nanocrystal Gels and Nanocomposites. ACS Macro Lett. 2012, 1, 1001–1006.
- Ma, T.; Lv, L.; Ouyang, C.; Hu, X.; Liao, X.; Song, Y.; Hu, X. Rheological Behavior and Particle Alignment of Cellulose Nanocrystal and Its Composite Hydrogels during 3D Printing. Carbohydr. Polym. 2021, 253, 117217.
- Trache, D. Nanocellulose as a Promising Sustainable Material for Biomedical Applications. AIMS Mater. Sci. 2018, 5, 201–205.
- Sultan, S.; Siqueira, G.; Zimmermann, T.; Mathew, A.P. 3D Printing of Nano-Cellulosic Biomaterials for Medical Applications. Curr. Opin. Biomed. Eng. 2017, 2, 29–34.
- Xiao, Y.; Liu, Y.Y.; Wang, Y.; Jin, Y.; Guo, X.; Liu, Y.Y.; Qi, X.; Lei, H.; Xu, H. Heat-Induced Whey Protein Isolate Gels Improved by Cellulose Nanocrystals: Gelling Properties and Microstructure. Carbohydr. Polym. 2020, 231, 115749.
- Boluk, Y.; Zhao, L.; Incani, V. Dispersions of Nanocrystalline Cellulose in Aqueous Polymer Solutions: Structure Formation of Colloidal Rods. Langmuir 2012, 28, 6114–6123.
- Hu, Z.; Cranston, E.D.; Ng, R.; Pelton, R. Tuning Cellulose Nanocrystal Gelation with Polysaccharides and Surfactants. Langmuir 2014, 30, 2684–2692.
- Lewis, L.; Derakhshandeh, M.; Hatzikiriakos, S.G.; Hamad, W.Y.; MacLachlan, M.J. Hydrothermal Gelation of Aqueous Cellulose Nanocrystal Suspensions. Biomacromolecules 2016, 17, 2747–2754.
- Shafeiei-Sabet, S.; Hamad, W.Y.; Hatzikiriakos, S.G. Influence of Degree of Sulfation on the Rheology of Cellulose Nanocrystal Suspensions. Rheol. Acta 2013, 52, 741–751.
- Shafiei-Sabet, S.; Hamad, W.Y.; Hatzikiriakos, S.G. Rheology of Nanocrystalline Cellulose Aqueous Suspensions. Langmuir 2012, 28, 17124–17133.
- Ureña-Benavides, E.E.; Kitchens, C.L. Wide-Angle X-ray Diffraction of Cellulose Nanocrystal−Alginate Nanocomposite Fibers. Macromolecules 2011, 44, 3478–3484.
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491.
- Dorris, A.; Gray, D.G. Gelation of Cellulose Nanocrystal Suspensions in Glycerol. Cellulose 2012, 19, 687–694.
- Moroni, L.; Burdick, J.A.; Highley, C.; Lee, S.J.; Morimoto, Y.; Takeuchi, S.; Yoo, J.J. Biofabrication Strategies for 3D in Vitro Models and Regenerative Medicine. Nat. Rev. Mater. 2018, 3, 21–37.
- Han, J.; Lei, T.; Wu, Q. Facile Preparation of Mouldable Polyvinyl Alcohol-Borax Hydrogels Reinforced by Well-Dispersed Cellulose Nanoparticles: Physical, Viscoelastic and Mechanical Properties. Cellulose 2013, 20, 2947–2958.
- McKee, J.R.; Appel, E.A.; Seitsonen, J.; Kontturi, E.; Scherman, O.A.; Ikkala, O. Healable, Stable and Stiff Hydrogels: Combining Conflicting Properties Using Dynamic and Selective Three-Component Recognition with Reinforcing Cellulose Nanorods. Adv. Funct. Mater. 2014, 24, 2706–2713.
- Zhou, C.; Wu, Q.; Lei, T.; Negulescu, I.I. Adsorption Kinetic and Equilibrium Studies for Methylene Blue Dye by Partially Hydrolyzed Polyacrylamide/Cellulose Nanocrystal Nanocomposite Hydrogels. Chem. Eng. J. 2014, 251, 17–24.
- Lin, N.; Gèze, A.; Wouessidjewe, D.; Huang, J.; Dufresne, A. Biocompatible Double-Membrane Hydrogels from Cationic Cellulose Nanocrystals and Anionic Alginate as Complexing Drugs Codelivery. ACS Appl. Mater. Interfaces 2016, 8, 6880–6889.
- Wang, K.; Nune, K.C.; Misra, R.D.K. The Functional Response of Alginate-Gelatin-Nanocrystalline Cellulose Injectable Hydrogels toward Delivery of Cells and Bioactive Molecules. Acta Biomater. 2016, 36, 143–151.
- Le Goff, K.J.; Gaillard, C.; Helbert, W.; Garnier, C.; Aubry, T. Rheological Study of Reinforcement of Agarose Hydrogels by Cellulose Nanowhiskers. Carbohydr. Polym. 2015, 116, 117–123.
- Sanna, R.; Fortunati, E.; Alzari, V.; Nuvoli, D.; Terenzi, A.; Casula, M.F.; Kenny, J.M.; Mariani, A. Poly(N-Vinylcaprolactam) Nanocomposites Containing Nanocrystalline Cellulose: A Green Approach to Thermoresponsive Hydrogels. Cellulose 2013, 20, 2393–2402.
- Gonzalez, J.S.; Ludueña, L.N.; Ponce, A.; Alvarez, V.A. Poly(Vinyl Alcohol)/Cellulose Nanowhiskers Nanocomposite Hydrogels for Potential Wound Dressings. Mater. Sci. Eng. C 2014, 34, 54–61.
- Abitbol, T.; Johnstone, T.; Quinn, T.M.; Gray, D.G. Reinforcement with Cellulose Nanocrystals of Poly(Vinyl Alcohol) Hydrogels Prepared by Cyclic Freezing and Thawing. Soft Matter 2011, 7, 2373.
- Aouada, F.A.; de Moura, M.R.; Orts, W.J.; Mattoso, L.H.C. Preparation and Characterization of Novel Micro- and Nanocomposite Hydrogels Containing Cellulosic Fibrils. J. Agric. Food Chem. 2011, 59, 9433–9442.
- Bajpai, S.K.; Pathak, V.; Soni, B.; Mohan, Y.M. CNWs Loaded Poly(SA) Hydrogels: Effect of High Concentration of CNWs on Water Uptake and Mechanical Properties. Carbohydr. Polym. 2014, 106, 351–358.
- Bajpai, S.K.; Pathak, V.; Chand, N.; Soni, B. Cellulose Nano Whiskers (CNWs) Loaded-Poly(Sodium Acrylate) Hydrogels. Part-I. Effect of Low Concentration of CNWs on Water Uptake. J. Macromol. Sci. Part A 2013, 50, 466–477.
- Karaaslan, M.A.; Tshabalala, M.A.; Yelle, D.J.; Buschle-Diller, G. Nanoreinforced Biocompatible Hydrogels from Wood Hemicelluloses and Cellulose Whiskers. Carbohydr. Polym. 2011, 86, 192–201.
- Spagnol, C.; Rodrigues, F.H.A.; Pereira, A.G.B.; Fajardo, A.R.; Rubira, A.F.; Muniz, E.C. Superabsorbent Hydrogel Composite Made of Cellulose Nanofibrils and Chitosan-Graft-Poly(Acrylic Acid). Carbohydr. Polym. 2012, 87, 2038–2045.
- Kelly, J.A.; Shukaliak, A.M.; Cheung, C.C.Y.; Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Responsive Photonic Hydrogels Based on Nanocrystalline Cellulose. Angew. Chem. Int. Ed. 2013, 52, 8912–8916.
- Yang, J.; Han, C.-R.; Duan, J.-F.; Xu, F.; Sun, R.-C. Mechanical and Viscoelastic Properties of Cellulose Nanocrystals Reinforced Poly(Ethylene Glycol) Nanocomposite Hydrogels. ACS Appl. Mater. Interfaces 2013, 5, 3199–3207.
- Yang, J.; Zhang, X.-M.; Xu, F. Design of Cellulose Nanocrystals Template-Assisted Composite Hydrogels: Insights from Static to Dynamic Alignment. Macromolecules 2015, 48, 1231–1239.
- Talantikite, M.; Gourlay, A.; Le Gall, S.; Cathala, B. Influence of Xyloglucan Molar Mass on Rheological Properties of Cellulose Nanocrystal/Xyloglucan Hydrogels. J. Renew. Mater. 2019, 7, 1381–1390.
- Chen, T.; Yang, Y.; Peng, H.; Whittaker, A.K.; Li, Y.; Zhao, Q.; Wang, Y.; Zhu, S.; Wang, Z. Cellulose Nanocrystals Reinforced Highly Stretchable Thermal-Sensitive Hydrogel with Ultra-High Drug Loading. Carbohydr. Polym. 2021, 266, 118122.
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial Hydrogels for Biomedical Applications. Heliyon 2020, 6, e03719.
- You, C.; Ning, L.; Wu, H.; Huang, C.; Wang, F. A Biocompatible and PH-Responsive Nanohydrogel Based on Cellulose Nanocrystal for Enhanced Toxic Reactive Oxygen Species Generation. Carbohydr. Polym. 2021, 258, 117685.
- Yao, Y.; Yu, S.; Shen, Y.; Wu, H. Facile Synthesis of Self-Dispersed β-Cyclodextrin-Coupled Cellulose Microgel for Sustained Release of Vanillin. Int. J. Biol. Macromol. 2022, 208, 70–79.
- Wu, J.; Lu, Q.; Wang, H.; Lu, B.; Huang, B. Controllable Construction of Temperature-Sensitive Supramolecular Hydrogel Based on Cellulose and Cyclodextrin. Polymers 2022, 14, 3801.
- Nath, P.C.; Debnath, S.; Sharma, M.; Sridhar, K.; Nayak, P.K.; Inbaraj, B.S. Recent Advances in Cellulose-Based Hydrogels: Food Applications. Foods 2023, 12, 350.
- Lin, N.; Dufresne, A. Supramolecular Hydrogels from In Situ Host–Guest Inclusion between Chemically Modified Cellulose Nanocrystals and Cyclodextrin. Biomacromolecules 2013, 14, 871–880.
- Park, E.; Ryu, J.H.; Lee, D.; Lee, H. Freeze–Thawing-Induced Macroporous Catechol Hydrogels with Shape Recovery and Sponge-like Properties. ACS Biomater. Sci. Eng. 2021, 7, 4318–4329.
- Guo, Y.; Wu, M.; Li, R.; Cai, Z.; Zhang, H. Thermostable Physically Crosslinked Cryogel from Carboxymethylated Konjac Glucomannan Fabricated by Freeze-Thawing. Food Hydrocoll. 2022, 122, 107103.
- De France, K.J.; Chan, K.J.W.; Cranston, E.D.; Hoare, T. Enhanced Mechanical Properties in Cellulose Nanocrystal–Poly(Oligoethylene Glycol Methacrylate) Injectable Nanocomposite Hydrogels through Control of Physical and Chemical Cross-Linking. Biomacromolecules 2016, 17, 649–660.
- Yang, J.; Zhao, J.-J.; Han, C.-R.; Duan, J.-F.; Xu, F.; Sun, R.-C. Tough Nanocomposite Hydrogels from Cellulose Nanocrystals/Poly(Acrylamide) Clusters: Influence of the Charge Density, Aspect Ratio and Surface Coating with PEG. Cellulose 2014, 21, 541–551.
- Ooi, S.Y.; Ahmad, I.; Amin, M.C.I.M. Cellulose Nanocrystals Extracted from Rice Husks as a Reinforcing Material in Gelatin Hydrogels for Use in Controlled Drug Delivery Systems. Ind. Crops Prod. 2016, 93, 227–234.
- Palaganas, N.B.; Mangadlao, J.D.; de Leon, A.C.C.; Palaganas, J.O.; Pangilinan, K.D.; Lee, Y.J.; Advincula, R.C. 3D Printing of Photocurable Cellulose Nanocrystal Composite for Fabrication of Complex Architectures via Stereolithography. ACS Appl. Mater. Interfaces 2017, 9, 34314–34324.
- Sultan, S.; Mathew, A.P. 3D Printed Scaffolds with Gradient Porosity Based on a Cellulose Nanocrystal Hydrogel. Nanoscale 2018, 10, 4421–4431.
- Zhou, C.; Wu, Q.; Yue, Y.; Zhang, Q. Application of Rod-Shaped Cellulose Nanocrystals in Polyacrylamide Hydrogels. J. Colloid Interface Sci. 2011, 353, 116–123.
- Zhou, C.; Wu, Q.; Zhang, Q. Dynamic Rheology Studies of in Situ Polymerization Process of Polyacrylamide–Cellulose Nanocrystal Composite Hydrogels. Colloid Polym. Sci. 2011, 289, 247–255.
- Hebeish, A.; Farag, S.; Sharaf, S.; Shaheen, T.I. Thermal Responsive Hydrogels Based on Semi Interpenetrating Network of Poly(NIPAm) and Cellulose Nanowhiskers. Carbohydr. Polym. 2014, 102, 159–166.
- Eyley, S.; Thielemans, W. Surface Modification of Cellulose Nanocrystals. Nanoscale 2014, 6, 7764–7779.
- Mihranyan, A. Viscoelastic Properties of Cross-Linked Polyvinyl Alcohol and Surface-Oxidized Cellulose Whisker Hydrogels. Cellulose 2013, 20, 1369–1376.
- Mauricio, M.R.; da Costa, P.G.; Haraguchi, S.K.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Synthesis of a Microhydrogel Composite from Cellulose Nanowhiskers and Starch for Drug Delivery. Carbohydr. Polym. 2015, 115, 715–722.
- Dash, R.; Foston, M.; Ragauskas, A.J. Improving the Mechanical and Thermal Properties of Gelatin Hydrogels Cross-Linked by Cellulose Nanowhiskers. Carbohydr. Polym. 2013, 91, 638–645.
- Li, W.; Lan, Y.; Guo, R.; Zhang, Y.; Xue, W.; Zhang, Y. In Vitro and In Vivo Evaluation of a Novel Collagen/Cellulose Nanocrystals Scaffold for Achieving the Sustained Release of Basic Fibroblast Growth Factor. J. Biomater. Appl. 2015, 29, 882–893.
- García-Astrain, C.; González, K.; Gurrea, T.; Guaresti, O.; Algar, I.; Eceiza, A.; Gabilondo, N. Maleimide-Grafted Cellulose Nanocrystals as Cross-Linkers for Bionanocomposite Hydrogels. Carbohydr. Polym. 2016, 149, 94–101.
- Yang, J.; Zhao, J.-J.; Xu, F.; Sun, R.-C. Revealing Strong Nanocomposite Hydrogels Reinforced by Cellulose Nanocrystals: Insight into Morphologies and Interactions. ACS Appl. Mater. Interfaces 2013, 5, 12960–12967.
- Yang, J.; Han, C.-R.; Duan, J.-F.; Ma, M.-G.; Zhang, X.-M.; Xu, F.; Sun, R.-C. Synthesis and Characterization of Mechanically Flexible and Tough Cellulose Nanocrystals–Polyacrylamide Nanocomposite Hydrogels. Cellulose 2013, 20, 227–237.
- Yang, J.; Zhao, J.-J.; Zhang, X.-M. Modification of Cellulose Nanocrystal-Reinforced Composite Hydrogels: Effects of Co-Crosslinked and Drying Treatment. Cellulose 2014, 21, 3487–3496.
- Atifi, S.; Su, S.; Hamad, W.Y. Mechanically Tunable Nanocomposite Hydrogels Based on Functionalized Cellulose Nanocrystals. Nord. Pulp Pap. Res. J. 2014, 29, 95–104.
- Yang, D.; Peng, X.; Zhong, L.; Cao, X.; Chen, W.; Wang, S.; Liu, C.; Sun, R. Fabrication of a Highly Elastic Nanocomposite Hydrogel by Surface Modification of Cellulose Nanocrystals. RSC Adv. 2015, 5, 13878–13885.
- Yang, J.; Han, C.-R.; Zhang, X.-M.; Xu, F.; Sun, R.-C. Cellulose Nanocrystals Mechanical Reinforcement in Composite Hydrogels with Multiple Cross-Links: Correlations between Dissipation Properties and Deformation Mechanisms. Macromolecules 2014, 47, 4077–4086.
- Chau, M.; De France, K.J.; Kopera, B.; Machado, V.R.; Rosenfeldt, S.; Reyes, L.; Chan, K.J.W.; Förster, S.; Cranston, E.D.; Hoare, T.; et al. Composite Hydrogels with Tunable Anisotropic Morphologies and Mechanical Properties. Chem. Mater. 2016, 28, 3406–3415.
- Köhnke, T.; Elder, T.; Theliander, H.; Ragauskas, A.J. Ice Templated and Cross-Linked Xylan/Nanocrystalline Cellulose Hydrogels. Carbohydr. Polym. 2014, 100, 24–30.
- Domingues, R.M.A.; Silva, M.; Gershovich, P.; Betta, S.; Babo, P.; Caridade, S.G.; Mano, J.F.; Motta, A.; Reis, R.L.; Gomes, M.E. Development of Injectable Hyaluronic Acid/Cellulose Nanocrystals Bionanocomposite Hydrogels for Tissue Engineering Applications. Bioconjug. Chem. 2015, 26, 1571–1581.
- Yang, X.; Bakaic, E.; Hoare, T.; Cranston, E.D. Injectable Polysaccharide Hydrogels Reinforced with Cellulose Nanocrystals: Morphology, Rheology, Degradation, and Cytotoxicity. Biomacromolecules 2013, 14, 4447–4455.
- Yang, J.; Han, C.; Xu, F.; Sun, R. Simple Approach to Reinforce Hydrogels with Cellulose Nanocrystals. Nanoscale 2014, 6, 5934.
- Ritonga, H.; Basri, M.; Rembon, F.; Ramadhan, L.O.A.; Nurdin, M. High Performance of Chitosan-Co-Polyacrylamide-TiO2 Crosslinked Glutaraldehyde Hydrogel as Soil Conditioner for Soybean Plant (Glycine max). Soil Sci. Annu. 2020, 71, 194–204.
- Cho, H.; Yoo, W.-J.; Ahn, J.; Chun, S.-J.; Lee, S.-Y.; Gwon, J. Preparation and Characterization of Cellulose Nanocrystals Reinforced Poly (Vinyl Alcohol) Based Hydrogels for Drug Delivery System. J. Korean Wood Sci. Technol. 2020, 48, 431–449.
- Zhu, Q.; Teng, J.; Liu, X.; Lan, Y.; Guo, R. Preparation and Characterization of Gentamycin Sulfate-Impregnated Gelatin Microspheres/Collagen–Cellulose/Nanocrystal Scaffolds. Polym. Bull. 2018, 75, 77–91.
- Liu, H.; Li, C.; Wang, B.; Sui, X.; Wang, L.; Yan, X.; Xu, H.; Zhang, L.; Zhong, Y.; Mao, Z. Self-Healing and Injectable Polysaccharide Hydrogels with Tunable Mechanical Properties. Cellulose 2018, 25, 559–571.
- Mihranyan, A.; Edsman, K.; Strømme, M. Rheological Properties of Cellulose Hydrogels Prepared from Cladophora Cellulose Powder. Food Hydrocoll. 2007, 21, 267–272.
- Shin, M.; Shin, S.-H.; Lee, M.; Kim, H.J.; Jeong, J.H.; Choi, Y.H.; Oh, D.X.; Park, J.; Jeon, H.; Eom, Y. Rheological Criteria for Distinguishing Self-Healing and Non-Self-Healing Hydrogels. Polymer 2021, 229, 123969.
- Hou, K.; Li, Y.; Liu, Y.; Zhang, R.; Hsiao, B.S.; Zhu, M. Continuous Fabrication of Cellulose Nanocrystal/Poly(Ethylene Glycol) Diacrylate Hydrogel Fiber from Nanocomposite Dispersion: Rheology, Preparation and Characterization. Polymer 2017, 123, 55–64.
- Zhou, L.; He, H.; Li, M.C.; Song, K.; Cheng, H.N.; Wu, Q. Morphological Influence of Cellulose Nanoparticles (CNs) from Cottonseed Hulls on Rheological Properties of Polyvinyl Alcohol/CN Suspensions. Carbohydr. Polym. 2016, 153, 445–454.
This entry is offline, you can click here to edit this entry!
