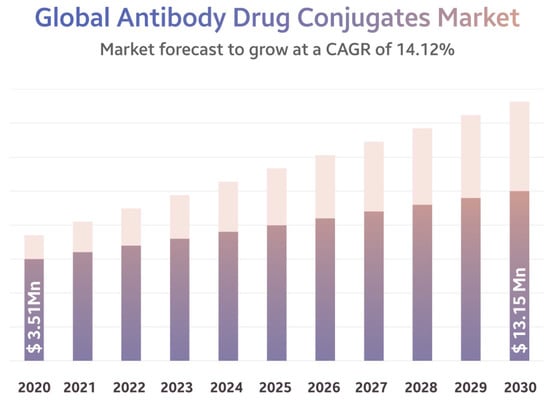
The cytotoxic effect and therapeutic window of mAbs by constructing antibody–drug conjugates (ADCs), in which the targeting moiety is the mAb that is linked to a highly toxic drug. According to a report from mid of last year, the global ADCs market accounted for USD 1387 million in 2016 and was worth USD 7.82 billion in 2022. It is estimated to increase in value to USD 13.15 billion by 2030. One of the critical points is the linkage of any substituent to the functional group of the mAb. Increasing the efficacy against cancer cells’ highly cytotoxic molecules (warheads) are connected biologically. The connections are completed by different types of linkers, or there are efforts to add biopolymer-based nanoparticles, including chemotherapeutic agents.
- antibody–drug conjugate (ADC)
- nanomedicine
- development
- market
- drug
1. Introduction
2. The Market of ADCs
| ADC Drug | Maker | Disease Indication | Payload/Payload Class | Target | mAb | Linker | Approval Year |
|---|---|---|---|---|---|---|---|
| Mirvetuximab soravtansine | ImmunoGen | Platinum-resistant ovarian cancer | Maytansinoid DM4 | FRα | IgG1 | / | 2022 |
| Tisotumab vedotin-tftv | Seagen Inc | Recurrent or metastatic cervical cancer | MMAE/auristatin | Tissue factor | IgG1 | Enzyme-cleavable | 2021 |
| Loncastuximab tesirine-lpyl | ADC Therapeutics | Large B-cell lymphoma | SG3199/PBD dimer | CD19 | IgG1 | Enzyme-cleavable | 2021 |
| Belantamab mafodotin-blmf | GlaxoSmithKline (GSK) | Adult patients with relapsed or refractory multiple myeloma | MMAF/auristatin | BCMA | IgG1 | Non-cleavable | 2020, withdrawn on 22 November, 2022 |
| Sacituzumab govitecan | Immunomedics | Adult patients with metastatic triple-negative breast cancer (mTNBC) who have received at least two prior therapies for patients with relapsed or refractory metastatic disease | SN-38/camptothecin | TROP2 | IgG1 | Acid-cleavable | 2020 |
| Trastuzumab deruxtecan | AstraZeneca/Daiichi Sankyo | Adult patients with unresectable or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 based regimens | DXd/camptothecin | HER2 | IgG1 | Enzyme-cleavable | 2019 |
| Enfortumab vedotin | Astellas/Seagen Genetics | Adult patients with locally advanced or metastatic urothelial cancer who have received a PD-1 or PD-L1 inhibitor and a Pt-containing therapy | MMAE/auristatin | Nectin4 | IgG1 | Enzyme-cleavable | 2019 |
| Polatuzumab vedotin-piiq | Genentech, Roche | Relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) | MMAE/auristatin | CD79 | IgG1 | Enzyme-cleavable | 2019 |
| Moxetumomab pasudotox | Astrazeneca | Adults with relapsed or refractory hairy cell leukemia (HCL) | PE38 (Pseudotox) | CD22 | IgG1 | Cleavable | 2018 |
| Inotuzumab ozogamicin | Pfizer/Wyeth | Relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia | Ozogamicin/calicheamicin | CD22 | IgG4 | Acid-cleavable | 2017 |
| Trastuzumab emtansine | Genentech, Roche | HER2-positive metastatic breast cancer (mBC) following treatment with trastuzumab and a maytansinoid | DM1/maytansinoid | HER2 | IgG1 | Nnon-cleavable | 2013 |
| Brentuximab vedotin | Seagen Genetics, Millennium/Takeda | Relapsed HL and relapsed sALCL | MMAE/auristatin | CD30 | IgG1 | Enzyme-cleavable | 2011 |
| Gemtuzumab ozogamicin | Pfizer/Wyeth | Relapsed acute myelogenous leukemia (AML) | Ozogamicin/calicheamicin | CD33 | IgG4 | Acid-cleavable | 2017; 2000 |
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15061761
References
- Fernald, K.D.S.; Pennings, H.P.G.; Bosch, J.F.V.D.; Commandeur, H.R.; Claassen, E. The moderating role of absorptive capacity and the differential effects of acquisitions and alliances on Big Pharma firms’ innovation performance. PLoS ONE 2017, 12, e0172488.
- Bayer ADC Fails Pivotal Mesothelioma Trial. Available online: https://www.fiercebiotech.com/biotech/bayer-adc-fails-pivotal-mesothelioma-trial (accessed on 24 July 2017).
- Pfizer Invests $43 Billion to Battle Cancer. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-invests-43-billion-battle-cancer (accessed on 13 March 2023).
- With $43B Buyout, Pfizer Sees Cancer Specialist Seagen as a ‘Goose’ Laying ‘Golden Eggs’. Available online: https://www.fiercepharma.com/pharma/43b-buyout-pfizer-sees-seagen-its-golden-goose (accessed on 13 March 2023).
- After Dose De-Escalation, Death Drives Magenta to Pause Antibody-Drug Conjugate Leukemia Trial. Available online: https://www.fiercebiotech.com/biotech/after-dose-de-escalation-death-drives-magenta-pause-antibody-drug-conjugate-leukemia-trial (accessed on 26 January 2023).
- Zolot, R.S.; Basu, S.; Million, R.P. Antibody–drug conjugates. Nat. Rev. Drug Discov. 2013, 12, 259–260.
- Kapinos, K.A.; Hu, E.; Trivedi, J.; Geethakumari, P.R.; Kansagra, A. Cost-Effectiveness Analysis of CAR T-Cell Therapies vs Antibody Drug Conjugates for Patients with Advanced Multiple Myeloma. Cancer Control. 2023, 30, 10732748221142945.
- Mckertish, C.M.; Kayser, V. Advances and Limitations of Antibody Drug Conjugates for Cancer. Biomedicines 2021, 9, 872.
- Wu, M.; Huang, W.; Yang, N.; Liu, Y. Learn from antibody–drug conjugates: Consideration in the future construction of peptide-drug conjugates for cancer therapy. Exp. Hematol. Oncol. 2022, 11, 93.
- Espelin, C.W.; Leonard, S.C.; Geretti, E.; Wickham, T.J.; Hendriks, B.S. Dual HER2 Targeting with Trastuzumab and Liposomal-Encapsulated Doxorubicin (MM-302) Demonstrates Synergistic Antitumor Activity in Breast and Gastric Cancer. Cancer Res. 2016, 76, 1517–1527.
- Hu, X.; Kwon, N.; Yan, K.; Sedgwick, A.C.; Chen, G.; He, X.; James, T.D.; Yoon, J. Bio-Conjugated Advanced Materials for Targeted Disease Theranostics. Adv. Funct. Mater. 2020, 30, 1907906.
- Rodallec, A.; Franco, C.; Robert, S.; Sicard, G.; Giacometti, S.; Lacarelle, B.; Bouquet, F.; Savina, A.; Lacroix, R.; Dignat-George, F.; et al. Prototyping Trastuzumab Docetaxel Immunoliposomes with a New FCM-Based Method to Quantify Optimal Antibody Density on Nanoparticles. Sci. Rep. 2020, 10, 4147.
- Matusewicz, L.; Filip-Psurska, B.; Psurski, M.; Tabaczar, S.; Podkalicka, J.; Wietrzyk, J.; Ziółkowski, P.; Czogalla, A.; Sikorski, A.F. EGFR-targeted immunoliposomes as a selective delivery system of simvastatin, with potential use in treatment of triple-negative breast cancers. Int. J. Pharm. 2019, 569, 118605.
- Juan, A.; Cimas, F.J.; Bravo, I.; Pandiella, A.; Ocaña, A.; Alonso-Moreno, C. Antibody Conjugation of Nanoparticles as Therapeutics for Breast Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 6018.
- Kasenda, B.; König, D.; Manni, M.; Ritschard, R.; Duthaler, U.; Bartoszek, E.; Bärenwaldt, A.; Deuster, S.; Hutter, G.; Cordier, D.; et al. Targeting immunoliposomes to EGFR-positive glioblastoma. ESMO Open 2022, 7, 100365.
- Mamot, C.; Wicki, A.; Hasler-Strub, U.; Riniker, S.; Li, Q.; Holer, L.; Bärtschi, D.; Zaman, K.; von Moos, R.; Dedes, K.J.; et al. A multicenter phase II trial of anti-EGFR-immunoliposomes loaded with doxorubicin in patients with advanced triple negative breast cancer. Sci. Rep. 2023, 13, 3705.
- Kumar, A.; Lale, S.V.; Alex, M.A.; Choudhary, V.; Koul, V. Folic Acid and Trastuzumab Conjugated Redox Responsive Random Multiblock Copolymeric Nanocarriers for Breast Cancer Therapy: In-Vitro and in-Vivo Studies. Colloids Surf. B Biointerfaces 2017, 149, 369–378.
- Peng, J.; Chen, J.; Xie, F.; Bao, W.; Xu, H.; Wang, H.; Xu, Y.; Du, Z. Herceptin-Conjugated Paclitaxel Loaded PCL-PEG Worm-like Nanocrystal Micelles for the Combinatorial Treatment of HER2-Positive Breast Cancer. Biomaterials 2019, 222, 119420.
- Kolahkaj, F.F.; Derakhshandeh, K.; Khaleseh, F.; Azandaryani, A.H.; Mansouri, K.; Khazaei, M. Active Targeting Carrier for Breast Cancer Treatment: Monoclonal Antibody Conjugated Epirubicin Loaded Nanoparticle. J. Drug Deliv. Sci. Technol. 2019, 53, 101136.
- Wang, Y.; Qian, J.; Yang, M.; Xu, W.; Wang, J.; Hou, G.; Ji, L.; Suo, A. Doxorubicin/Cisplatin Co-Loaded Hyaluronic Acid/Chitosan-Based Nanoparticles for in Vitro Synergistic Combination Chemotherapy of Breast Cancer. Carbohydr. Polym. 2019, 225, 115206.
- Zhong, S.; Ling, Z.; Zhou, Z.; He, J.; Ran, H.; Wang, Z.; Zhang, Q.; Song, W.; Zhang, Y.; Luo, J. Herceptin-Decorated Paclitaxel-Loaded Poly(Lactide-Co-Glycolide) Nanobubbles: Ultrasound-Facilitated Release and Targeted Accumulation in Breast Cancers. Pharm. Dev. Technol. 2020, 25, 454–463.
- Pilkington, G.A.; Pedersen, J.S.; Briscoe, W.H. Dendrimer nanofluids in the concentrated regime: From polymer melts to soft spheres. Langmuir 2015, 31, 3333–3342.
- Choi, J.-H.; Gu, H.-J.; Park, K.-H.; Hwang, D.-S.; Kim, G.-C. Anti-Cancer Activity of the Combinational Treatment of Noozone Cold Plasma with p-FAK Antibody-Conjugated Gold Nanoparticles in OSCC Xenograft Mice. Biomedicines 2022, 10, 2259.
- Lodhi, M.S.; Khalid, F.; Khan, M.T.; Samra, Z.Q.; Muhammad, S.; Zhang, Y.-J.; Mou, K. A Novel Method of Magnetic Nanoparticles Functionalized with Anti-Folate Receptor Antibody and Methotrexate for Antibody Mediated Targeted Drug Delivery. Molecules 2022, 27, 261.
- Wilcock, P.; Webster, R.M. The breast cancer drug market. Nat. Rev. Drug Discov. 2021, 20, 339–340.
- Antibody Drug Conjugate Market, a $13.15 billion Industry by 2030 with a CAGR of 14.12%. Available online: https://www.globenewswire.com/en/news-release/2022/06/21/2465821/0/en/Antibody-Drug-Conjugate-Market-a-13-15-billion-Industry-by-2030-with-a-CAGR-of-14-12.html (accessed on 21 June 2022).
- Pazo, C.D.; Nawaz, K.; Webster, R.M. The oncology market for antibody–drug conjugates. Nat. Rev. Drug Discov. 2021, 20, 583–584.
- Antibody-Drug Conjugates (ADCs) List Approved by FDA (2000–2023). Available online: https://axispharm.com/antibody-drug-conjugatesadcs-list-approved-by-fda2000-2022/ (accessed on 27 December 2022).
