Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Catalysis on TiO2 nanomaterials in the presence of H2O and oxygen plays a crucial role in the advancement of many different fields, such as clean energy technologies, catalysis, disinfection, and bioimplants. Photocatalysis on TiO2 nanomaterials is well-established and has advanced in the last decades in terms of the understanding of its underlying principles and improvement of its efficiency.
- reactive oxygen species
- photocatalytic efficiency
- charge separation
1. Reactive Oxygen Species in TiO2 Photocatalysis
ROS can be considered primary intermediates of photocatalytic reactions with these four recognized as the main ones: hydroxyl radical (·OH), superoxide anion radical (·O2−), hydrogen peroxide (H2O2), and singlet oxygen (1O2) [1][2]. ROS seem to form mainly from the interaction of the VB hole with molecules (such as H2O) or species, oxidizing the latter and typically resulting in ·OH centers [3][4][5]. This could also happen in hole-trapping processes in TiO2, such as at bridging O2−, resulting in the formation mainly of ·O− (“deprotonated ·OH”) [6][7][8]. Because of the high potential barrier of free ·OH for desorption, adsorbed ·OH is considered more favorable and is usually equated to trapped holes due to the adsorption–desorption equilibrium [1][6].
A detailed summary of generating the four major ROS on the TiO2 surface can be viewed in terms of bridging and terminal OH sites [3]. The reactions occurring at the anatase and rutile are differentiated by the arrow lines (double lines are restricted to anatase), whereas broken lines refer to adsorption/desorption. At the bridged OH site, a photogenerated hole attacks the O2− bridge (step a), forming Ti-O· and Ti-OH (step b), which can be reversed by recombining with an electron from the CB. Some surface-trapped holes at the anatase can be released as ·OH into the solution. At the rutile surface with its suitable distance between adjacent Ti surface atoms, a different scenario occurs. Once another hole is formed in the same trapped hole-containing particle, the hole could migrate and interact with the existing hole resulting in a peroxo-bridged structure at the surface (step c). Further reactions of these structures could then generate other ROS [1][3].
At the terminal OH site, a photogenerated electron can interact and is trapped at the Ti4+ site, transforming it to Ti3+ (step b) [9]. The trapped electron in the Ti3+ could then reduce oxygen to form an ·O2− (adsorbed) (step d) (which, with further reduction, could become an adsorbed H2O2 or as (Ti)-OOH (step c)). The H2O2 that is adsorbed could also be reoxidized to produce an adsorbed ·O2−, which can be desorbed to return to the initial state (step a). As the peroxo bond needs to be dissociated, the production of ·OH is highly unlikely when the adsorbed H2O2 is being reduced [1][3]. These schemes also show a sensible explanation for the influence of the adsorption of H2O2 in forming ROS, which has been well-considered for increasing the photocatalytic performance of TiO2.
Adsorbed ROS can also have a more direct impact on photocatalytic performance [1]. Anatase and rutile show different reactivity towards forming ·OH and ·O2− [10][11], likely due to their H2O2 adsorption [8] in addition to their band edge alignment. One-step oxidation of H2O2 produces ·O2−, which is more remarkable for anatase, whereas one-step reduction produces ·OH, which is only observed for rutile or rutile-containing forms and is believed to be due to the structure of the adsorbed H2O2 on rutile vs. anatase [12].
2. Nanomorphologies and Structural States of TiO2
Due to its wide applicability, TiO2 has been produced via different means, with the resulting TiO2 structural polymorphs—i.e., anatase, rutile, or brookite, among others [13]—and morphology being highly influenced by the preparation method. The different TiO2 morphologies add to the variety of properties and performance exhibited by TiO2. In addition to bulk TiO2 [14][15][16][17], in recent decades, TiO2 nanomaterials of various morphologies have also been developed, resulting in the current plethora of TiO2 nanomorphologies (Figure 1). These have been synthesized using different means for various targeted applications, achieving a range of photocatalytic efficiencies. Note that some morphologies are preferably prepared using certain procedures (e.g., sol-gel method for nanopowders and anodization for nanotubes), whereas some procedures (e.g. hydrothermal synthesis) can be used and modified to produce various morphologies (such as nanospindles, nanorhombus, nanorods, or nanosheets).
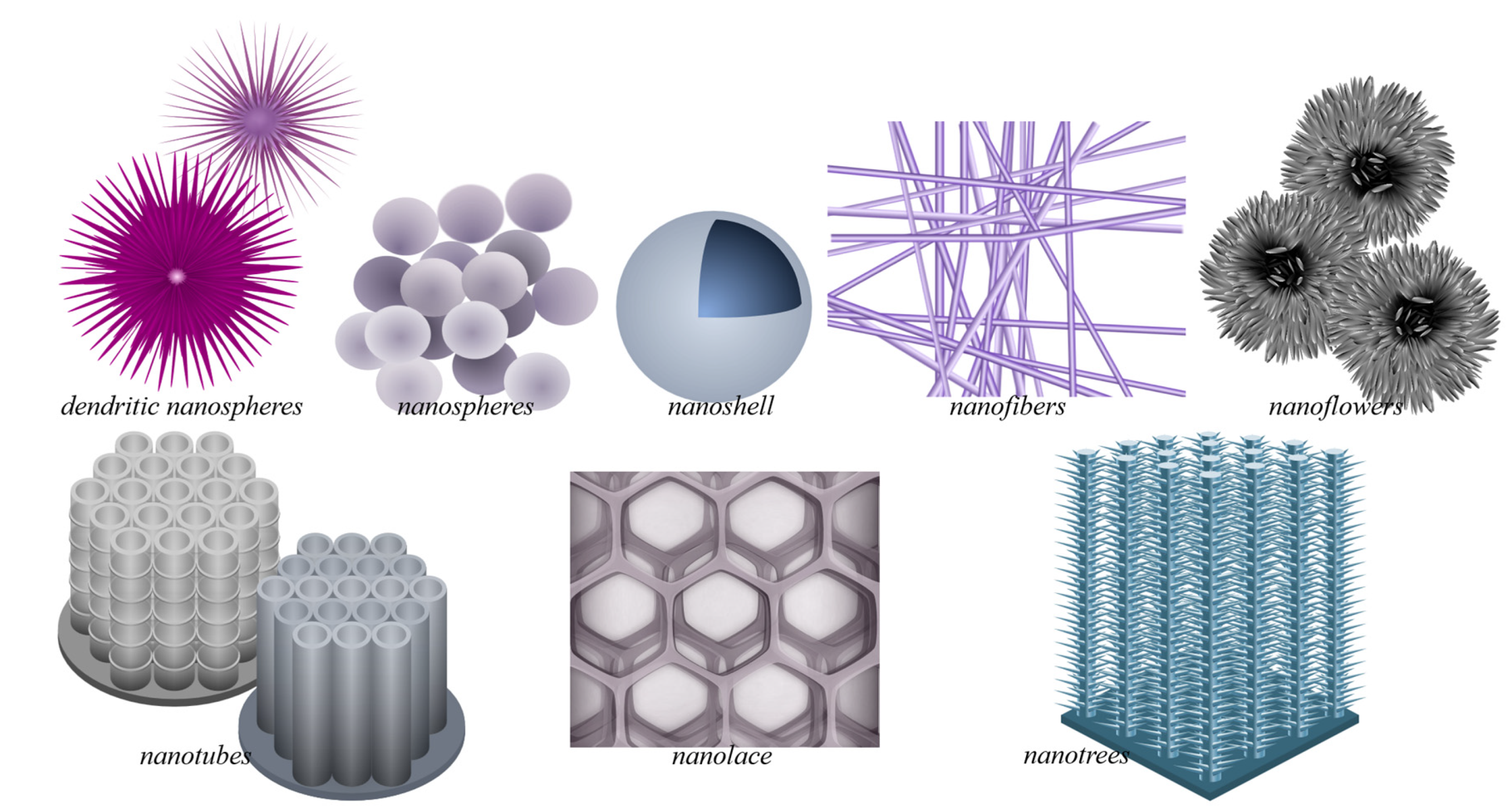
Figure 1. Plethora of TiO2 nanostructures. This includes free-standing nanomaterials, such as TiO2 dendritic nanospheres, nanospheres, nanoshells, nanofibers, and nanoflowers, and nanostructures grown on bulk substrates, such as nanotubes, nanolace, and nanotrees. Nanomaterials present increased catalytic activity due to their interesting properties (increased surface area, enhanced charge separation, light absorption/harvesting), which are desirable in catalytic TiO2 applications. For image reprint/adaptation, please cite the original source: Querebillo, C.J. A Review on Nano Ti-Based Oxides for Dark and Photocatalysis: From Photoinduced Processes to Bioimplant Applications. Nanomaterials 2023, 13(6), 982; https://doi.org/10.3390/nano13060982. Copyright 2023 Querebillo, C.J.
The morphologies of nanosized titanium dioxide can be grouped according to their dimension classifications: zero-dimensional (0D) includes nanopowders, nanocrystals, and quantum dots (QD); one-dimensional (1D) includes nanowires, nanofibers, nanotubes, and nanorods; two-dimensional (2D) includes nanosheets; and three-dimensional (3D) includes nanotube arrays. Mixed morphologies, such as nanosheets with QDs, also exist.
Many studies on photocatalysis have been conducted on nanoparticle suspensions [18][19][20], which are inconvenient and result in practical difficulties [21] because complete photocatalyst recovery is challenging. Therefore, studies have resorted to photocatalyst immobilization [22][23][24][25], which, however, requires catalysts of high activity. TiO2 of different nanomorphologies were used, evaluated, and/or compared in terms of performance [26][27][28]. The photocatalytic performance reported, usually measured by dye degradation rate, varies and depends on the experimental setup/condition, such as the illumination and probe used, though the typical pseudo-first-order rate constant k is within the 10−3–10−1 min−1 range. Therefore, studies also sometimes include a reference TiO2, such as commercially available P25 for benchmarking. Nevertheless, from this summary, one also sees that generally, some improvement in photocatalytic performance is brought about by nanomorphology based on the obtained k values being mainly in the 10−1–10−2 min−1 range for systems with varied morphology, which are at least one order of magnitude better than the usual for those with nanopowders (k~10−2–10−3 min−1).
Post-synthesis heat treatment (calcination/annealing) mainly dictates the structural state. Heat treatment can be performed to transform the amorphous state to rutile (≥600 °C) or anatase (300 °C to 500 °C) or to transform anatase to the more stable rutile [29]. The crystalline state also influences photocatalytic activity. It is commonly agreed that anatase is better than the other states (such as rutile) due to its higher surface affinity and slower recombination rate. However, mixed states (such as the case of P25) also exhibit good photocatalytic activity, though the surface crystalline state seems to play a more crucial role in such cases of mixed states due to the fact that photocatalytic reactions take place at the surface [30].
3. Photocatalytic Disinfection Using TiO2 Nanostructures
The photocatalytic ROS production on TiO2 NPs, while it may pose some health risks, can also provide benefits. As early as 1985, the photocatalytic microbicidal effect of TiO2 has been reported by Matsunaga et al. [31]. More studies have then been carried out on the bacteria-killing action of TiO2 [32][33][34][35]. Maness et al. [32] attribute this effect to the lipid peroxidation in the microbe (in their case, in E. coli) due to the photocatalytic oxidative property of TiO2 NPs. Upon initiation of lipid peroxidation by ROS, propagation can happen via the generation of peroxy radical intermediates, which can also react with other lipid molecules. Superoxide radical could also be involved, as it can also be photogenerated on TiO2. This can react with an intermediate hydroperoxide to form new reaction chains that can go through the damaged cell membrane. Once the cell wall is broken down, TiO2 NPs themselves could also possibly directly attack the cell membrane [32]. It is important to remember though that the microbe’s response to photocatalytic disinfection action can also be influenced by its level of protective enzymes against oxidative stress [34].
It is generally accepted that the photocatalytic antibacterial properties of TiO2 are due to its ROS formation, whereby ·OH is thought to play a crucial role [34]. Yet, novel TiO2-based materials also point to the role and use of other ROS. The development of different TiO2 nanostructures paves the way for advancements in photocatalytic disinfection on TiO2, and some examples, also in relation to the formation of ROS, are presented here.
Nanocomposites made from TiO2 NPs on Si nanostructured surfaces can be used as antibacterial surfaces for dental and orthopedic implants, and the TiO2 NPs themselves can be spray-coated to surfaces for disinfection of microbes upon irradiation [11]. The nanostructures are said to rupture the bacterial cell wall, whereas the ROS from TiO2 NPs can oxidize organic matter (such as bacteria) to prevent bacterial growth [36], which could eventually form biofilms. On the other hand, the free radicals photogenerated on TiO2 can also disrupt and destroy biofilms. This is important since killing bacteria within a biofilm is quite challenging; the biofilm shields the bacteria from antibiotics, antibodies, and immune cells [37][38]. Using TiO2 exposed to UVA, the biofilm formation of P. aerigunosa was inhibited via ROS attack to disrupt the bacterial cell membrane (disabling bacteria to form biofilm), and the ε-poly(L-lysine) of the cells already in the biofilm was weakened [11]. Through a plasma electrolytic oxidation process, Nagay and coworkers produced N- and Bi-doped TiO2 coatings that kill bacteria because of the generation of ROS upon visible light exposure [39][40].
Efforts to extend photosensitization with TiO2 were performed by forming composites with materials such as MoS2 and l-arginine and/or doping with Yb and Er [39][41]. However, the broader spectral range or near-IR sensitization is usually brought about by the additional component (and not by TiO2). On the other hand, other works utilize the photogenerated charge carriers from TiO2 and enhance the catalytic effect by the addition of other components for antibacterial purposes. For example, TiO2 combined with graphdiyne (GDY) was synthesized into nanofibers by electrostatic force to produce ROS and prolong the antibacterial effect. When exposed to light, electrons and holes are generated on the TiO2 and GDY surface, with the photogenerated electrons of TiO2 being easily transferrable to the GDY surface. There, ·OH and ·O2− are formed because they can react easily with water and O2. The extended lifetimes of the charge carriers enhance ROS generation and the resulting bactericidal effect. Overall, these processes inhibit the methicillin-resistant S. aureus (MRSA) biofilm formation and promote the regeneration of bone tissues [11][39][42].
In general, the photocatalytic disinfection by TiO2 nanocomposite antimicrobial coatings entails the incorporation of inorganic metals/nonmetals (such as Ag, Cu, Mn, P, Ca, and F) and/or 2D materials (graphydiyne, MXenes, and metal–organic frameworks, etc.) into TiO2 to control the porosity of the surface, crystallinity, charge transfer, and disinfecting property against critical pathogens, such as S. aureus and E. coli but also H1N1, vesicular stomatitis virus (the safe surrogate virus for SARS-CoV-2), and the human coronavirus HCoV-NL63 [11]. Such light-catalyzed coatings could prevent microbes from reactivating to completely destroy them, and with the high mobility of ROS in the air, airborne microbes could also be targeted [43]. The high interest in the inactivation and disinfection of coronaviruses emerged recently due to the recent pandemic. Some of these studies were on the photocatalytic disinfection of coronavirus using TiO2 NP coatings, with the mechanism attributed to their generation of ROS [11][44][45]. For interest in antimicrobial coatings, readers are referred to Kumaravel et al. [11].
4. Efforts to Improve the Photocatalytic Efficiency of TiO2 Nanomaterials
The photocatalytic activity of TiO2 nanomaterials improves by resorting to different morphologies. Their nanosize alone increases the surface area, providing more active sites. Additionally, due to the interesting properties afforded by its size, the use of nanomaterials also improves photocatalytic efficiency by enhancing charge separation and light harvesting and increasing the surface-to-bulk ratio. These improvements are presented in this section.
4.1. Enhanced Charge Separation
The increased photocatalytic performance of TiO2 nanoparticles cannot be attributed to the increased specific surface area alone but also to the increase in the surface-to-bulk ratio with decreasing particle size. The latter results in shorter diffusion pathways that the charge carriers have to traverse to reach the surface, which is the photocatalytic reaction site [46]. Adding adsorbed species further provides electron/hole scavengers that could also improve the charge separation [46][47][48]. The decrease in the size though also blueshifts the TiO2 absorption edge [49] and could result in unstable NPs [50]. Therefore, an optimized size is needed to balance the properties in terms of charge separation, light absorption, and stability. During thermal treatment, which is typically needed for good crystallinity and increased photocatalytic performance, aggregation could occur, and this can be prevented by preparing highly-dispersed TiO2 clusters (such as those synthesized with zeolites [51]), which also shows high photocatalytic activity. These spatially separated TiO2 species were also prepared as single-site catalysts that also show high photocatalytic electron–hole pair reactivity and selectivity [6]. The high photocatalytic reactivity observed for highly dispersed TiO2 species is attributed to the highly selective formation of a longer-lived (up to µs), localized charge-transfer excited state compared to that of bulk TiO2 (ns) [46].
The aggregate formation is not always disadvantageous. The mechanisms portrayed for a single semiconductor NP are simplified, and a more accurate representation would consider that TiO2 NPs have the tendency to self-aggregate in aqueous solution to form a 3D framework. This happens by aligning their atomic planes with each other, allowing for efficient charge carrier transport without interfacial trap interferences in a so-called “antenna effect”. Through this, the photogenerated excitons in a nanoparticle will be transported throughout the network until they get trapped individually in a suitable site (e.g., via a redox reaction with an adsorbed electron acceptor/donor on one particle in the network). Charge carriers that are not trapped continue to traverse through the network until they themselves react. Therefore, through forming 3D aggregates, better electron mobility is achieved for TiO2 particles [6][52].
When the aggregates align, they act as if they are an array of nanowires that facilitate efficient CT throughout the network. In fact, 1D morphologies, such as TiO2 nanowires, nanotubes, and nanorods, are hailed for their efficient electron transport since the photoexcited charge carriers could move along the length, increasing delocalization and resulting in long diffusion lengths (>200 nm), which delays the charge recombination and prevents electrons from residing in traps [53][54][55][56][57][58]. For example, using TiO2 nanotube arrays (TNAs) for PEC water splitting can improve the photocatalytic efficiency and result in a photocurrent of up to 10 times since loss of photogenerated electrons is prevented with the electrons being able to diffuse along the tube towards the collecting substrate. The improved performance using TNA is said to not only come from enhanced charge separation and better electron transport due to the orderly arrangement [53][59][60][61] of this 3D structure but also due to better light harvesting [46][53].
Meanwhile, the photocatalytic activity of pure TiO2 nanomaterials is considerably small and requires mostly UV light due to its bandgap. Hence, various attempts were conducted to improve its efficiency and increase its absorption range. Doping is one widely used approach, and this has been performed with transition metal ions, such as Fe3+, which increased the efficiency for pollutant photodecomposition likely due to the fact that it decreases the Eg and broadens the absorption range to the visible region [6][62]. Oxygen doping in TiO2 interstices can also improve photocatalytic performance by enhancing charge separation efficiency. For the photodegradation of methyl orange, O-doped or oxidative TiO2 showed a 2~3.7 times higher rate than pristine TiO2, with the former fabricated using KMnO4 to create trap sites to separate charges via bandgap impurity states [63].
Depositing other catalysts has also been another approach. For example, a noble metal, such as Pt, Au, and Ru, can be deposited to increase the photocatalytic activity of TiO2 towards the decomposition of organics and photocatalytic water splitting [6][48][64][65][66]. This enhanced activity is likely due to improved charge separation as bulk electrons transfer to the metal and therefore to the surface of TiO2 [6][64][65][66][67]. Though not all photogenerated electrons transfer from the titania to the metal, the enhanced separation of photogenerated charge carriers increases the electron lifetime in TiO2. The separation is likely enhanced by the surface plasmon resonance (SPR) of the metal and the resulting increased localized EM field caused by the exposure of the metal to light. This induces charge carrier formation near the TiO2 surface, with which carriers can easily reach surface sites and improve charge separation. Loading TiO2 with gold instead of platinum also extends the absorption of TiO2 to the visible range up to near-infrared [6][68][69][70][71]. Using gold cores with TiO2 shells also exhibits remarkable photocatalytic activity compared to TiO2 due to enhanced separation of photogenerated charge carriers with the gold core serving as an electron trap [72].
In addition to metals, metal oxides can also be deposited on TiO2 to help improve charge separation and photocatalysis. For example, a Pd-NiO/TiO2 catalyst has been prepared to improve photocatalytic CO2 reduction. Due to the high electron density needed to drive this multielectron reduction, a p–n junction formed by introducing NiO to TiO2 helps to drive hole transport to NiO, whereas the Pd forming a Schottky junction with TiO2 facilitates semiconductor-to-metal electron transfer. These migrations towards NiO and Pd enhance the charge separation and result in high electron density around Pd, which can be used to transform CO2 efficiently and selectively to CH4 by reduction [73].
The coupling of the semiconductor to TiO2 can also be achieved by coupling it with another TiO2 phase. As mentioned before, mixed-phase TiO2 of anatase and rutile demonstrates improved photocatalytic performance than by using only pure anatase or rutile. This could be due to the formation of heterojunction when their valence and conduction band edges come into contact [11][74]. Several models were proposed to explain this synergistic effect in mixed phases. First was the model proposed by Bickley et al. based on the positions of the CBs of anatase and rutile in relation to each other [75]. Separation of charges then happens in anatase and trapping of an electron occurs in the rutile phase. In another “spatial charge-separation model,” Hurum and coworkers propose that the opposite is happening such that electrons are transferred from the rutile CB to a trapping site in anatase [76].
Meanwhile, the “interfacial model” proposed by Nair et al. looks at the band bending at the interface between anatase and rutile. The electron transfer should occur from the anatase to the rutile upon UV illumination due to the CB energy of the anatase being more negative than that of the rutile. When the illumination is with λ > 380 nm, the rutile is activated, and its CB shifts upward due to accumulated photoinduced electrons enabling the electrons in the rutile to reach the CB of anatase [46]. Thus, the “interfacial model” presents a directional movement of the electrons depending on whether the irradiation is ≤ or >380 nm and upon consideration of the interfacial band bending [77]. One can expect that the interfacial nanostructure plays a role in the electron transfer between the components and therefore in the overall photocatalytic performance. Further discussion on the advantages of mixed-phase TiO2 for photocatalysis can be found in the literature [46].
Fe2O3 has also been combined with TiO2 via photodeposition to enhance charge separation for contaminant decomposition and PEC water splitting. The achieved enhancement of more than 200% in complete mineralization kinetics was ascribed to the transfer of photoelectrons from the TiO2 to the Fe2O3, which in turn favors the rate-determining step of oxygen reduction [78]. Graphitic nanocarbon has also been added to TiO2 nanomaterials to improve charge separation. By covering short single-wall carbon nanotubes (SWCNT) (~125 nm) around TiO2 NPs (100 nm) using a hydration-condensation technique, longer lifetimes of photogenerated charge carrier and improved photocatalytic activity for the degradation of an aldehyde was achieved. This was better than nanographene and longer SWCNT hybrid systems. The shorter SWCNT provides greater interfacial contact with each TiO2 NP, more electron transport channels, and more efficient shuttling of electrons from TiO2 NP to SWCNT, delaying charge recombination. Improved SWCNT debundling with the short ones also affords these advantages to a larger portion of the composite [79].
The semiconductor junction can also be quite complex, involving materials such as MXenes. For example, Biswal and coworkers designed a Ti3C2/N, S-TiO2/g-C3N4 heterojunction to boost the spatial separation of charges and their transport in light of a photocatalytic water-splitting application [79]. This heterostructure was produced by thermal annealing and ultrasonic-assisted impregnation for H2 production that is up to ~4-fold higher than pristine S-doped titania. The dual heterojunction formed (a n–n heterojunction with a Schottky junction) likely not only enables effective charge carrier separation as CT channels [79] but also reduces the band gap due to the adjustment of the energy bands. In(OH)3-TiO2 heterostructures were also formed for enhanced photocatalytic H2 evolution. The band-gap tuning and improved charge separation resulted in up to a >15-fold increase in activity compared to commercial P25 [80].
4.2. Enhanced Light Harvesting
The morphology or nanostructure of TiO2 also improves photocatalytic efficiency due to enhanced light harvesting. As mentioned above, nanoparticle aggregation (~500 nm) can improve light harvesting due to its high scattering effect, resulting in photon reabsorption. This increased visible-range absorption in turn increases the number of excited charges as seen in the increased current density. Such aggregates display unsmooth surfaces, resulting in better molecule adsorption in large surface areas and pore sizes [81].
Nanotextured TiO2 substrate produced by nanomolding also displays efficient light harvesting. The hierarchical nanopattern of dual-scale nanoscale craters featuring smaller bumps couples both the longer and shorter wavelengths of light resulting in a light trapping effect for efficient light utilization and at a wide angular range [82]. Similarly, cicada-wing-like structures were used as imprints to form nanohole structures of TiO2 decorated with Ag NPs (10−25 nm) for methylene blue (MB) photocatalytic degradation. The structure did not only exhibit extended absorption to the visible range but also greater light absorption, likely due to the SPR effect from Ag and the nanotexture of TiO2. This is based on the photocatalytic decomposition rate obtained for the Ag-TiO2 nanotexture being 2.7 times higher than the nanotextured TiO2 alone but more than 7 times higher than P25. This shows that even with just nanotextured TiO2, improved photocatalytic performance can be seen. As discussed in the previous section, the Schottky barrier formed between Ag and TiO2 could also improve the charge separation [83]. Hollow particles of TiO2 decorated with Au@Ag core–shell NPs also display enhanced light harvesting due to the combined strengths of the components of having a strong, broadened localized SPR, large specific surface area, and favorable light scattering properties [84].
Orderly arrays of nanostructures, such as TNA, serve as effective light scattering layers according to the Mie scattering theory. The Mie scattering effect displayed by anodized TNA or NPs has been used in solar cells to harvest more sunlight and enhance charge conduction [85]. Mie scattering is important in explaining particle size-dependent Raman enhancement observed with semiconductors [86][87] and is brought about by the plasmon resonance at the surface of the sphere causing signal enhancement that depends on the size as one comes closer to the lowest transverse electric mode of NPs. In addition to the Mie effect, size quantization also affects the Raman intensity obtained on TiO2 NPs [88].
The surface-enhanced Raman (SER) effect on semiconductors has also been well-observed [89][90][91][92][93], and the influence of nanostructuring on SER scattering, in particular on TiO2, has been investigated. Whereas CT and chemical contribution can provide an enhancement factor (EF) of ~103 [94][95], EM enhancement is also afforded in TiO2 nanostructures of a high aspect ratio, such as nanotubes and nanofibers [96][97][98][99]. Han et al. [98] showed concrete evidence of morphology-dependent EM enhancement using cyt b5 heme as an indirectly-attached SER probe to reduce the chemical contribution to the Raman signals. Using EM field calculations, the particle’s aspect ratio was shown to increase the “hot spots” (regions of enhanced EM field) at the TiO2-water interface [98], improving the structure’s light-harvesting ability. Hence, other morphologies of higher anisotropy, such as TiO2 nanotubes, were further studied, showing a similar morphology-dependent EM field enhancement [96][99]. TNAs were shown to exhibit different Raman enhancements depending on the tube length, which fits the EM field calculation showing hot spots along the nanotube length [96][99]. The TNA of high EM field enhancement was shown to perform better as a photocatalyst for visible-light-degradation of an azo dye pollutant immobilized on TNA. Interestingly, the TNA’s optical response (i.e., its EM field enhancement) correlates with the photocatalytic degradation rate occurring on it [100].
Similar to other TiO2 nanostructures (see above), incorporating other components to form nanocomposites with TiO2 nanotubes can further improve not only the charge separation but also the light-harvesting ability. For example, S-doping or the addition of CdS NPs to TiO2 nanotubes resulted in enhanced visible-light water splitting [101][102][103]. Ultrafine Pt NPs were also added into TiO2 nanotubes for the efficient photocatalytic formation of methane from carbon dioxide and water. The nanotubes allowed for a homogeneous distribution of Pt NPs, which accept electrons and become sites for reduction, thereby also allowing efficient separation of charges [46]. Furthermore, even structures obtained from TNA somehow retain the light enhancement afforded by the 2D periodic arrangement of the nanotubes. For example, nitridation of the TNA resulting in a partially collapsed nanotube structure of TiN also shows wavelength-dependent EM field enhancement and corresponding light enhancement [104].
The 2D periodic arrangement in TNAs enables them to behave as photonic crystals—with photonic lattices reflecting the light of certain wavelengths—bringing about localized EM field enhancement [96][99][105][106][107][108]. Interestingly, this photonic crystal-like character has also been observed in inverse-opal (IO) structures, which also achieved SER EF of around 104 (though likely due to both chemical and EM contributions) [96][99][105], and which can also be made from TiO2. IO TiO2 also shows promising performance as photocatalysts [109][110][111][112], with their light harvesting extended to the visible range [111][112] and their ability to generate slow photons [112][113][114]. The slow photons have been shown to significantly increase the interaction of TiO2 with light and can work synergistically to amplify the chemical enhancement in the catalyst [110].
4.3. Black TiO2
The photocatalytic efficiency of TiO2 nanomaterials can be improved by enhancing the separation of their charge carriers and improving their light harvesting and absorption properties. Therefore, having a material that encompasses both is an ultimate surface-engineering achievement in this regard. Black TiO2 ticks both requirements and reasonably has then become a hot topic in TiO2 photocatalysis in the last decade or so.
Though previous studies already describe a similar material, as indicated in reviews [115][116][117], all papers seem to point to the work of Chen et al. [118] for introducing (and coining the term) “black TiO2” to describe the partially hydrogenated titania nanocrystals which exhibit a reduced bandgap due to a disordered layer at the surface of its crystals. This material exhibited a redshifted absorption onset to near-infrared (compared to the starting TiO2 nanomaterial), which was not surprising considering its visible color change. That is, due to the hydrogenation process, the crystals changed from white to black (hence the name). Consequently, this also results in a decreased bandgap of ~2.18 eV, making black TiO2 a good catalyst for visible-light irradiation. Additionally, it also exhibits good stability, making it an ideal catalyst for use under continued irradiation. From calculations, it also presents localized photogenerated charge carriers, indicative of slowed-down recombination, which is beneficial for photocatalysis. This makes the work of Chen et al. the first reported use of black titania for photocatalytic purposes [116].
From then on, many studies have been carried out to synthesize, characterize, and evaluate the photocatalytic performance of black TiO2 nanomaterials. Different methods have been developed to reduce TiO2 without the use of high pressure, as was conducted in the work of Chen et al. [118]. These include (electro-)chemical reduction [119][120], solvothermal hydrogenation [121], thermal reduction [122], reduction at the solid phase (reductant + heat) [123], anodization (and annealing) [124], ultrasonication [125] plasma treatment [126], gel combustion [127], or a combination of these [128]. Most of these strategies are similar to the synthesis of TiO2 nanomaterials, with a reductant source/ reducing condition (either chemical, thermal, hydrogen, or reducing gases, such as hydrogen, nitrogen, and argon) added. Since black TiO2 is formed by the reduction of TiO2, it is also called “reduced TiO2” [129][130][131] or “hydrogenated TiO2” [132] and represented with the formula TiO2−x, the −x indicating the formation of oxygen vacancies [129][130].
What is interesting then is the concept of forming a novel material due to the introduction of surface defects, and yet, as this is also a TiO2 nanomaterial, it can also exist in different morphologies and structural states, resulting in a plethora of black TiO2 of various properties and photocatalytic performance. Table 1 gives examples of these materials and their photocatalytic performance in terms of organic compound degradation and hydrogen generation, with the latter being an important solar-driven application of black TiO2. Chen et al. [117] give a comprehensive review of black TiO2 nanomaterials, including their properties and examples of application, whereas Naldoni et al. [116] give a good summary of the photocatalytic H2 generation on black TiO2. The readers are encouraged to take a look at these reviews.
Table 1. Examples of black (and colored) TiO2 nanomaterials and their photocatalytic performance in terms of degradation of organic compounds or hydrogen evolution. For table reprint/adaptation, please cite the original source: Querebillo, C.J. A Review on Nano Ti-Based Oxides for Dark and Photocatalysis: From Photoinduced Processes to Bioimplant Applications. Nanomaterials 2023, 13(6), 982; https://doi.org/10.3390/nano13060982. Copyright 2023 Querebillo, C.J.
| Material | Degradation/Removal of Organics | H2 Generation | Reference Material/Comparison | Ref. |
|---|---|---|---|---|
| Black TiO2 nanocrystals/NPs | ~7.5× faster MB degradation, solar illumination | 0.1 ± 0.02 mmol h−1 g−1 (2 orders higher (solar simulator or visible IR light)) | Degradation: pristine TiO2 nanocrystals H2 generation: most semiconductor photocatalysts |
[118] |
| Up to k = 0.68 min−1 MO degradation, 2.4× faster (simulated sunlight) | Up to 5.2 mmol h−1g−1, 1.7× faster (simulated sunlight) | Pristine P25 degradation: k = 0.28 min−1 H2 generation: 5.2 mmol h−1 g−1 |
[123] | |
| Up to apparent k (kapp) = 0.998 h−1 or 0.0166 min−1 acetaminophen removal, 1.9× faster than P25 and 4.9× faster than sintered P25 (solar illum. AM 1.5G) | P25: k = 0.527 h−1 or 0.00878 min−1 Sintered P25: 0.203 h−1 or 0.00338 min−1 |
[133] | ||
| Estimate: ~15 µmol h−1 g−1 ~5−7× higher (AM 1.5 illum.; 100 mW cm−2) |
Anatase nanopowders; estimate: ~2−3 µmol h−1 g−1 | [134] | ||
| Anatase; ~1.5× faster, MB degradation finished in 18 min (solar illumination) | Degussa-P25: MB degradation finished in 18 min. (solar illumination) | [127] | ||
| Black hydroxylated TiO2 (ultrasonic.) | 5.8× (solar illumination) and 7.2× (visible light) faster acid fuchsin decomposition; amorphous state | Original sol TiO2 (non-ultrasonically processed) | [125] | |
| Black TiO2 nanotube array (TNA) | Estimate: 10~15% (3–4×) better photocatalytic degradation (brilliant blue KN-R dye; 175 W Xe lamp) | Pristine TNA | [120] | |
| Ordered mesoporous black TiO2 | 136.2 µmol h−1, ~2× higher (solar) |
Pristine mesoporous TiO2: 76 µmol h−1 | [122] | |
| Mesoporous black TiO2 hollow spheres | 241 µmol h−1 (0.1) g−1, ~2× higher (solar) | Black TiO2 NPs: 118 µmol h−1 (0.1) g−1 Mesoporous TiO2 hollow spheres: 81 µmol h−1 (0.1) g−1 |
[121] | |
| Defective black TiO2 (dimpled morphology, anodization) | High oxygen vacancy concentration (CVo): up to ~80% RhB degradation (after 4 h), ~1.3× better than low CVo and ~4× better than TNA. | Low CVo: up to 60% RhB degradation (after 4 h) TNA: ~20% RhB degradation (after 4 h) |
[124] | |
| Grey TiO2 nanoparticles (flow furnace) | Estimate: ~75 µmol h−1 g−1 ~25−37× higher (AM 1.5 illum.; 100 mW cm−2) |
Anatase nanopowders: estimate: ~2−3 µmol h−1 g−1 | [134] | |
| Grey TiO2 nanoparticles (hydrogen. at high P) | Estimate: ~80−85 µmol h−1 g−1 ~27−42× higher (AM 1.5 illum.; 100 mW cm−2) |
Anatase nanopowders: estimate: ~2−3 µmol h−1 g−1 | [134] | |
| Colored TiO2 (dark blue) |
Up to C/C0 = 0.14 (estimated k = 0.197 min−1), 1.4× faster MO degradation (300 W, Xe lamp UV–vis light) | Up to max. prod. of 6.5 mmol h−1 g−1, 7.2× higher (UV-vis light); ~180 µmol h−1 g−1 (vis-IR) | Pristine P25 Degradation: C/C0 = 0.24 (estimated k = 0.143 min−1) H2 generation: 0.9 mmol h−1 g−1 |
[119] |
| Blue TiO2(B) single-crystal nanorods | k = 0.0146 min−1; 97.01% RhB degradation (after 150 min), 6.9× and 2.1× better than TiO2 NPs and TiO2 NRs, respectively (vis light); 98.56% deg. RhB (solar light), 99.12% deg. Phenol (solar); reaction constant (krxn) = 0.0250 (RhB) and 0.0366 (phenol), 8.8× higher than TiO2 NPs | Up to 149. µmol h−1 g−1 (AM 1.5 illumination), ~26.6× higher than TiO2 NPs | Degradation: TiO2 NPs: k = 0.0016 min−1; 14.06% RhB degradation (after 150 min, vis light) TiO2 NRs: k = 0.0053 min−1; 46.44% RhB degradation (after 150 min, vis light) H2 evolution: TiO2 NPs: 5.6 µmol h−1 g−1 (AM 1.5 illumination) TiO2 NRs: 40.8 µmol h−1 g−1 (AM 1.5 illumination) |
[135] |
Some examples included in Table 1 are of different color naming (termed “colored TiO2”), such as green, grey, and blue TiO2 [116][119][132][134][135]. This is based on the understanding that the visual colors exhibited by TiO2 are brought about by intrinsic defects, such as due to the presence of Ti3+ and/or oxygen vacancies [116][119][124][132][135] or by doping with impurities [116][126]. Such defects create extra electronic states in the TiO2 bandgap, i.e., intraband gap states, which alters the optoelectronic properties of TiO2 [116]. Whether this is also the case for the color of black TiO2 is still a controversy. While some reports claim that the formation of these color-inducing intrinsic defects in TiO2 results from the hydrogenation [129], with the color depending on the extent of reduction and reducing condition [132], others propose that the black color is due to the disordered surface [118][126]. The disordered surface is caused by hydrogen and allows hydrogen to swiftly navigate around and induce electronic structural changes [136]. Midgap band states are formed because of the changes in the structure [127] brought about by the excessive lattice disorder. They can form an extended energy state by overlapping with the edge of the conduction band and also possibly combining with the valence band [126].
An effort to further unravel the relationship between the defect nature and photocatalytic activity of reduced TiO2 was performed by Will et al. [132] by considering that the intrinsic defects created on the surface are pertinent to the photocatalytic process and the location of the defect depends on the structural state and reducing conditions. They found that the introduction of Ti3+ at the surface results in a surface with substoichiometry, which activates the surface for photocatalysis. However, too long hydrogenation or too much Ti3+ is detrimental to its activity, as these provide additional recombination sites or prevent efficient interfacial CT. Surface roughness and strain were also not important for the activation of photocatalysis.
The photocatalytic activity of black TiO2 nanomaterial can be further enhanced by forming appropriate heterojunctions with other materials [132], a similar strategy used with TiO2 nanomaterials. Further, amorphous black TiO2 can also be synthesized and used for photocatalysis [125], which is important for applications such as for bone implants. Black TiO2 was shown to exhibit biocompatibility [137], regenerative properties [138], photothermal properties [137][138][139], and microbicidal action [137][139][140][141], among others. As such, black TiO2 shows promise for cancer treatment [138][139], as a bone implant coating, and for disinfection purposes [137][139][140][141]. Similar to TiO2, the photo(electro)catalytic disinfection with black TiO2 nanomaterials is also claimed to occur due to ROS, in particular, superoxide and/or hydroxyl radicals [140][141]. Nevertheless, the biosafety of black TiO2 needs to be further studied to intensify its application in the biomedical field.
This entry is adapted from the peer-reviewed paper 10.3390/nano13060982
References
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336.
- Jedsukontorn, T.; Meeyoo, V.; Saito, N.; Hunsom, M. Effect of Electron Acceptors H2O2 and O2 on the Generated Reactive Oxygen Species 1O2 and OH· in TiO2-Catalyzed Photocatalytic Oxidation of Glycerol. Cuihua Xuebao/Chin. J. Catal. 2016, 37, 1975–1981.
- Nosaka, Y.; Nosaka, A. Understanding Hydroxyl Radical (•OH) Generation Processes in Photocatalysis. ACS Energy Lett. 2016, 1, 356–359.
- Gao, R.; Stark, J.; Bahnemann, D.W.; Rabani, J. Quantum Yields of Hydroxyl Radicals in Illuminated TiO2 Nanocrystallite Layers. J. Photochem. Photobiol. A Chem. 2002, 148, 387–391.
- Diesen, V.; Jonsson, M. Formation of H2O2 in TiO2 Photocatalysis of Oxygenated and Deoxygenated Aqueous Systems: A Probe for Photocatalytically Produced Hydroxyl Radicals. J. Phys. Chem. C 2014, 118, 10083–10087.
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986.
- Lawless, D.; Serpone, N.; Meisel, D. Role of OH· Radicals and Trapped Holes in Photocatalysis. A Pulse Radiolysis Study. J. Phys. Chem. 1991, 95, 5166–5170.
- Zhang, J.; Nosaka, Y. Mechanism of the OH Radical Generation in Photocatalysis with TiO2 of Different Crystalline Types. J. Phys. Chem. C 2014, 118, 10824–10832.
- Liao, H.; Reitberger, T. Generation of Free OHaq Radicals by Black Light Illumination of Degussa (Evonik) P25 TiO2 Aqueous Suspensions. Catalysts 2013, 3, 418–443.
- Pillai, S.C.; Periyat, P.; George, R.; Mccormack, D.E.; Seery, M.K.; Hayden, H.; Colreavy, J.; Corr, D.; Hinder, S.J. Synthesis of High-Temperature Stable Anatase TiO2 Photocatalyst. J. Phys. Chem. C 2007, 111, 1605–1611.
- Kumaravel, V.; Nair, K.M.; Mathew, S.; Bartlett, J.; Kennedy, J.E.; Manning, H.G.; Whelan, B.J.; Leyland, N.S.; Pillai, S.C. Antimicrobial TiO2 Nanocomposite Coatings for Surfaces, Dental and Orthopaedic Implants. Chem. Eng. J. 2021, 416, 129071.
- Kakuma, Y.; Nosaka, A.Y.; Nosaka, Y. Difference in TiO2 Photocatalytic Mechanism between Rutile and Anatase Studied by the Detection of Active Oxygen and Surface Species in Water. Phys. Chem. Chem. Phys. 2015, 17, 18691–18698.
- Fu, Z.; Liang, Y.; Wang, S.; Zhong, Z. Structural Phase Transition and Mechanical Properties of TiO2 under High Pressure. Phys. Status Solidi Basic Res. 2013, 250, 2206–2214.
- Rich, C.C.; Knorr, F.J.; McHale, J.L. Trap State Photoluminescence of Nanocrystalline and Bulk TiO2: Implications for Carrier Transport. Mater. Res. Soc. Symp. Proc. 2010, 1268, 117–122.
- Kurtz, R.L.; Stock-Bauer, R.; Msdey, T.E.; Román, E.; De Segovia, J.L. Synchrotron Radiation Studies of H2O Adsorption on TiO2(110). Surf. Sci. 1989, 218, 178–200.
- Tôrres, A.R.; Azevedo, E.B.; Resende, N.S.; Dezotti, M. A Comparison between Bulk and Supported TiO2 Photocatalysts in the Degradation of Formic Acid. Braz. J. Chem. Eng. 2007, 24, 185–192.
- Ma, H.; Lenz, K.A.; Gao, X.; Li, S.; Wallis, L.K. Comparative Toxicity of a Food Additive TiO2, a Bulk TiO2, and a Nano-Sized P25 to a Model Organism the Nematode C. Elegans. Environ. Sci. Pollut. Res. 2019, 26, 3556–3568.
- Hosseinnia, A.; Keyanpour-Rad, M.; Pazouki, M. Photo-Catalytic Degradation of Organic Dyes with Different Chromophores by Synthesized Nanosize TiO2 Particles. World Appl. Sci. J. 2010, 8, 1327–1332.
- Shrivastava, V.S. Photocatalytic Degradation of Methylene Blue Dye and Chromium Metal from Wastewater Using Nanocrystalline TiO2 Semiconductor. Arch. Appl. Sci. Res. 2012, 4, 1244–1254.
- Joshi, K.M.; Shrivastava, V.S. Degradation of Alizarine Red-S (A Textiles Dye) by Photocatalysis Using ZnO and TiO2 as Photocatalyst. Int. J. Environ. Sci. 2011, 2, 8–21.
- Tayade, R.J.; Surolia, P.K.; Kulkarni, R.G.; Raksh, V.; Tayade, R.J.; Surolia, P.K.; Kulkarni, R.G.; Jasra, R.V. Photocatalytic Degradation of Dyes and Organic Contaminants in Water Using Nanocrystalline Anatase and Rutile TiO2. Sci. Technol. Adv. Mater. 2007, 8, 455–462.
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications and Applications. Chem. Rev. 2007, 107, 2891–2959.
- Torimoto, T.; Ito, S.; Kuwabata, S.; Yoneyama, H. Effects of Adsorbents Used as Supports for Titanium Dioxide Loading on Photocatalytic Degradation of Propyzamide. Environ. Sci. Technol. 1996, 30, 1275–1281.
- Fox, M.A.; Doan, K.E.; Dulay, M.T. The Effect of the “Inert” Support on Relative Photocatalytic Activity in the Oxidative Decomposition of Alcohols on Irradiated Titanium Dioxide Composites. Res. Chem. Intermed. 1994, 20, 711–721.
- Rachel, A.; Subrahmanyam, M.; Boule, P. Comparison of Photocatalytic Efficiencies of TiO2 in Suspended and Immobilised Form for the Photocatalytic Degradation of Nitrobenzenesulfonic Acids. Appl. Catal. B Environ. 2002, 37, 301–308.
- Yu, J.C.; Yu, J.; Zhao, J. Enhanced Photocatalytic Activity of Mesoporous and Ordinary TiO2 thin Films by Sulfuric Acid Treatment. Appl. Catal. B Environ. 2002, 36, 31–43.
- Wang, J.A.; Limas-Ballesteros, R.; López, T.; Moreno, A.; Gómez, R.; Novaro, O.; Bokhimi, X. Quantitative Determination of Titanium Lattice Defects and Solid-State Reaction Mechanism in Iron-Doped TiO2 Photocatalysts. J. Phys. Chem. B 2001, 105, 9692–9698.
- Yan, J.; Wu, G.; Guan, N.; Li, L.; Li, Z.; Cao, X. Understanding the Effect of Surface/Bulk Defects on the Photocatalytic Activity of TiO2: Anatase versus Rutile. Phys. Chem. Chem. Phys. 2013, 15, 10978–10988.
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176.
- Zhang, H.; Yu, M. Photocatalytic Activity of TiO2 Nanofibers: The Surface Crystalline Phase Matters. Nanomaterials 2019, 9, 535.
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical Sterilization of Microbial Cells by Semiconductor Powders. FEMS Microbiol. Lett. 1985, 29, 211–214.
- Maness, P.C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal Activity of Photocatalytic TiO2 Reaction: Toward an Understanding of Its Killing Mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098.
- Saito, T.; Iwase, T.; Horie, J.; Morioka, T. Mode of Photocatalytic Bactericidal Action of Powdered Semiconductor TiO2 on Mutans Streptococci. J. Photochem. Photobiol. B Biol. 1992, 14, 369–379.
- Robertson, P.K.J.; Robertson, J.M.C.; Bahnemann, D.W. Removal of Microorganisms and Their Chemical Metabolites from Water Using Semiconductor Photocatalysis. J. Hazard. Mater. 2012, 211–212, 161–171.
- Wei, C.; Lin, W.Y.; Zainal, Z.; Williams, N.E.; Zhu, K.; Kruzlc, A.P.; Smith, R.L.; Rajeshwar, K. Bactericidal Activity of TiO2 Photocatalyst in Aqueous Media: Toward a Solar-Assisted Water Disinfection System. Environ. Sci. Technol. 1994, 28, 934–938.
- Singh, J.; Hegde, P.B.; Avasthi, S.; Sen, P. Scalable Hybrid Antibacterial Surfaces: TiO2 Nanoparticles with Black Silicon. ACS Omega 2022, 7, 7816–7824.
- Kalelkar, P.P.; Riddick, M.; García, A.J. Biomaterial-Based Antimicrobial Therapies for the Treatment of Bacterial Infections. Nat. Rev. Mater. 2022, 7, 39–54.
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322.
- Lu, X.; Wu, Z.; Xu, K.; Wang, X.; Wang, S.; Qiu, H.; Li, X.; Chen, J. Multifunctional Coatings of Titanium Implants Toward Promoting Osseointegration and Preventing Infection: Recent Developments. Front. Bioeng. Biotechnol. 2021, 9, 783816.
- Nagay, B.E.; Dini, C.; Cordeiro, J.M.; Ricomini-Filho, A.P.; De Avila, E.D.; Rangel, E.C.; Da Cruz, N.C.; Barão, V.A.R. Visible-Light-Induced Photocatalytic and Antibacterial Activity of TiO2 Codoped with Nitrogen and Bismuth: New Perspectives to Control Implant-Biofilm-Related Diseases. ACS Appl. Mater. Interfaces 2019, 11, 18186–18202.
- Han, X.; Zhang, G.; Chai, M.; Zhang, X. Light-Assisted Therapy for Biofilm Infected Micro-Arc Oxidation TiO2 Coating on Bone Implants. Biomed. Mater. 2021, 16, 025018.
- Wang, R.; Shi, M.; Xu, F.; Qiu, Y.; Zhang, P.; Shen, K.; Zhao, Q.; Yu, J.; Zhang, Y. Graphdiyne-Modified TiO2 Nanofibers with Osteoinductive and Enhanced Photocatalytic Antibacterial Activities to Prevent Implant Infection. Nat. Commun. 2020, 11, 4465.
- Horváth, E.; Rossi, L.; Mercier, C.; Lehmann, C.; Sienkiewicz, A.; Forró, L. Photocatalytic Nanowires-Based Air Filter: Towards Reusable Protective Masks. Adv. Funct. Mater. 2020, 30, 2004615.
- Khaiboullina, S.; Uppal, T.; Dhabarde, N.; Subramanian, V.R.; Verma, S.C. Inactivation of Human Coronavirus by Titania Nanoparticle Coatings and Uvc Radiation: Throwing Light on Sars-CoV-2. Viruses 2021, 13, 19.
- Yoshizawa, N.; Ishihara, R.; Omiya, D.; Ishitsuka, M.; Hirano, S.; Suzuki, T. Application of a Photocatalyst as an Inactivator of Bovine Coronavirus. Viruses 2020, 12, 1372.
- He, H.; Liu, C.; Dubois, K.D.; Jin, T.; Louis, M.E.; Li, G. Enhanced Charge Separation in Nanostructured TiO2 Materials for Photocatalytic and Photovoltaic Applications. Ind. Eng. Chem. Res. 2012, 51, 11841–11849.
- Kočí, K.; Obalová, L.; Matějová, L.; Plachá, D.; Lacný, Z.; Jirkovský, J.; Šolcová, O. Effect of TiO2 Particle Size on the Photocatalytic Reduction of CO2. Appl. Catal. B Environ. 2009, 89, 494–502.
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997.
- Satoh, N.; Nakashima, T.; Kamikura, K.; Yamamoto, K. Quantum Size Effect in TiO2 Nanoparticles Prepared by Finely Controlled Metal Assembly on Dendrimer Templates. Nat. Nanotechnol. 2008, 3, 106–111.
- Li, W.; Ni, C.; Lin, H.; Huang, C.P.; Shah, S.I. Size Dependence of Thermal Stability of TiO2 Nanoparticles. J. Appl. Phys. 2004, 96, 6663–6668.
- Jansson, I.; Suárez, S.; Garcia-Garcia, F.J.; Sánchez, B. Zeolite-TiO2 Hybrid Composites for Pollutant Degradation in Gas Phase. Appl. Catal. B Environ. 2015, 178, 100–107.
- Wang, C.Y.; Böttcher, C.; Bahnemann, D.W.; Dohrmann, J.K. A Comparative Study of Nanometer Sized Fe(III)-Doped TiO2 Photocatalysts: Synthesis, Characterization and Activity. J. Mater. Chem. 2003, 13, 2322–2329.
- Zhu, K.; Neale, N.R.; Miedaner, A.; Frank, A.J. Enhanced Charge-Collection Efficiencies and Light Scattering in Dye-Sensitized Solar Cells Using Oriented TiO2 Nanotubes Arrays. Nano Lett. 2007, 7, 69–74.
- De Jongh, P.E.; Vanmaekelbergh, D. Trap-Limited Electronic Transport in Assemblies of Nanometer-Size TiO2 Particles. Phys. Rev. Lett. 1996, 77, 3427–3430.
- Nelson, J.; Haque, S.A.; Klug, D.R.; Durrant, J.R. Trap-Limited Recombination in Dye-Sensitized Nanocrystalline Metal Oxide Electrodes. Phys. Rev. B 2001, 63, 205321-1–205321-29.
- Cao, F.; Oskam, G.; Meyer, G.J.; Searson, P.C. Electron Transport in Porous Nanocrystalline TiO2 Photoelectrochemical Cells. J. Phys. Chem. 1996, 100, 17021–17027.
- Dloczik, L.; Ileperuma, O.; Lauermann, I.; Peter, L.M.; Ponomarev, E.A.; Redmond, G.; Shaw, N.J.; Uhlendorf, I. Dynamic Response of Dye-Sensitized Nanocrystalline Solar Cells: Characterization by Intensity-Modulated Photocurrent Spectroscopy. J. Phys. Chem. B 1997, 5647, 10281–10289.
- Park, N.G.; Frank, A.J. Evaluation of the Charge-Collection Efficiency of Dye-Sensitized Nanocrystalline TiO2 Solar Cells. J. Phys. Chem. B 1999, 103, 782–791.
- Paulose, M.; Shankar, K.; Yoriya, S.; Prakasam, H.E.; Varghese, O.K.; Mor, G.K.; Latempa, T.A.; Fitzgerald, A.; Grimes, C.A. Anodic Growth of Highly Ordered TiO2 Nanotube Arrays to 134 µm in Length. J. Phys. Chem. B 2006, 110, 16179–16184.
- Ohsaki, Y.; Masaki, N.; Kitamura, T.; Wada, Y.; Okamoto, T.; Sekino, T.; Niihara, K.; Yanagida, S. Dye-Sensitized TiO2 Nanotube Solar Cells: Fabrication and Electronic Characterization. Phys. Chem. Chem. Phys. 2005, 7, 4157–4163.
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of Highly-Ordered TiO2 Nanotube Arrays in Dye-Sensitized Solar Cells. Nano Lett. 2006, 6, 215–218.
- Bahnemann, D.W. Current Challenges in Photo Catalysis: Improved Photocatalysts and Appropriate Photoreactor Engineering. Res. Chem. Intermed. 2000, 26, 207–220.
- Qin, Y.; Deng, L.; Wei, S.; Bai, H.; Gao, W.; Jiao, W.; Yu, T. An Effective Strategy for Improving Charge Separation Efficiency and Photocatalytic Degradation Performance Using a Facilely Synthesized Oxidative TiO2 Catalyst. Dalt. Trans. 2022, 51, 6899–6907.
- Furube, A.; Asahi, T.; Masuhara, H.; Yamashita, H.; Anpo, M. Direct Observation of a Picosecond Charge Separation Process in Photoexcited Platinum-Loaded TiO2 Particles by Femtosecond Diffuse Reflectance Spectroscopy. Chem. Phys. Lett. 2001, 336, 424–430.
- Yang, L.; Gao, P.; Lu, J.; Guo, W.; Zhuang, Z.; Wang, Q.; Li, W.; Feng, Z. Mechanism Analysis of Au, Ru Noble Metal Clusters Modified on TiO2(101) to Intensify Overall Photocatalytic Water Splitting. RSC Adv. 2020, 10, 20654–20664.
- Kmetykó, Á.; Szániel, Á.; Tsakiroglou, C.; Dombi, A.; Hernádi, K. Enhanced Photocatalytic H2 Generation on Noble Metal Modified TiO2 Catalysts Excited with Visible Light Irradiation. React. Kinet. Mech. Catal. 2016, 117, 379–390.
- Chiarello, G.L.; Aguirre, M.H.; Selli, E. Hydrogen Production by Photocatalytic Steam Reforming of Methanol on Noble Metal-Modified TiO2. J. Catal. 2010, 273, 182–190.
- Nie, J.; Schneider, J.; Sieland, F.; Zhou, L.; Xia, S.; Bahnemann, D.W. New Insights into the Surface Plasmon Resonance (SPR) Driven Photocatalytic H2 Production of Au-TiO2. RSC Adv. 2018, 8, 25881–25887.
- Singh, Y.; Raghuwanshi, S.K. Titanium Dioxide (TiO2) Coated Optical Fiber-Based SPR Sensor in near-Infrared Region with Bimetallic Structure for Enhanced Sensitivity. Optik 2021, 226, 165842.
- Lin, Z.; Wang, X.; Liu, J.; Tian, Z.; Dai, L.; He, B.; Han, C.; Wu, Y.; Zeng, Z.; Hu, Z. On the Role of Localized Surface Plasmon Resonance in UV-Vis Light Irradiated Au/TiO2 Photocatalysis Systems: Pros and Cons. Nanoscale 2015, 7, 4114–4123.
- Tian, Y.; Tatsuma, T. Mechanisms and Applications of Plasmon-Induced Charge Separation at TiO2 Films Loaded with Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637.
- Wu, L.; Ma, S.; Chen, P.; Li, X. The Mechanism of Enhanced Charge Separation and Photocatalytic Activity for 2 Core-Shell Nanocomposite. Int. J. Environ. Anal. Chem. 2023, 103, 201–211.
- Lan, D.; Pang, F.; Ge, J. Enhanced Charge Separation in NiO and PdCo-Modified TiO2 Photocatalysts for Efficient and Selective Photoreduction of CO2. ACS Appl. Energy Mater. 2021, 4, 6324–6332.
- Cao, F.; Xiong, J.; Wu, F.; Liu, Q.; Shi, Z.; Yu, Y.; Wang, X.; Li, L. Enhanced Photoelectrochemical Performance from Rationally Designed Anatase/Rutile TiO2 Heterostructures. ACS Appl. Mater. Interfaces 2016, 8, 12239–12245.
- Bickley, R.I.; Gonzalez-Carreno, T.; Lees, J.S.; Palmisano, L.; Tilley, R.J.D. A Structural Investigation of Titanium Dioxide Photocatalysts. J. Solid State Chem. 1991, 92, 178–190.
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549.
- Nair, R.G.; Paul, S.; Samdarshi, S.K. High UV/Visible Light Activity of Mixed Phase Titania: A Generic Mechanism. Sol. Energy Mater. Sol. Cells 2011, 95, 1901–1907.
- Moniz, S.J.A.; Shevlin, S.A.; An, X.; Guo, Z.X.; Tang, J. Fe2O3-TiO2 Nanocomposites for Enhanced Charge Separation and Photocatalytic Activity. Chem.—A Eur. J. 2014, 20, 15571–15579.
- Al Mayyahi, A.; Everhart, B.M.; Shrestha, T.B.; Back, T.C.; Amama, P.B. Enhanced Charge Separation in TiO2/Nanocarbon Hybrid Photocatalysts through Coupling with Short Carbon Nanotubes. RSC Adv. 2021, 11, 11702–11713.
- Du, X.; Hu, J.; Xie, J.; Hao, A.; Lu, Z.; Cao, Y. Simultaneously Tailor Band Structure and Accelerate Charge Separation by Constructing Novel In(OH)3-TiO2 Heterojunction for Enhanced Photocatalytic Water Reduction. Appl. Surf. Sci. 2022, 593, 153305.
- Ge, Z.; Wang, C.; Chen, Z.; Wang, T.; Chen, T.; Shi, R.; Yu, S.; Liu, J. Investigation of the TiO2 Nanoparticles Aggregation with High Light Harvesting for High-Efficiency Dye-Sensitized Solar Cells. Mater. Res. Bull. 2021, 135, 111148.
- Ram, S.K.; Rizzoli, R.; Desta, D.; Jeppesen, B.R.; Bellettato, M.; Samatov, I.; Tsao, Y.C.; Johannsen, S.R.; Neuvonen, P.T.; Pedersen, T.G.; et al. Directly Patterned TiO2 Nanostructures for Efficient Light Harvesting in Thin Film Solar Cells. J. Phys. D Appl. Phys. 2015, 48, 365101.
- Zada, I.; Zhang, W.; Zheng, W.; Zhu, Y.; Zhang, Z.; Zhang, J.; Imtiaz, M.; Abbas, W.; Zhang, D. The Highly Efficient Photocatalytic and Light Harvesting Property of Ag-TiO2 with Negative Nano-Holes Structure Inspired from Cicada Wings. Sci. Rep. 2017, 7, 17277.
- Yun, J.; Hwang, S.H.; Jang, J. Fabrication of Core/Shell Nanoparticles Decorated TiO2 Hollow Structure for Efficient Light-Harvesting in Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 2055–2063.
- Yang, H.Y.; Rho, W.Y.; Lee, S.K.; Kim, S.H.; Hahn, Y.B. TiO2 Nanoparticles/Nanotubes for Efficient Light Harvesting in Perovskite Solar Cells. Nanomaterials 2019, 9, 326.
- Lombardi, J.R.; Birke, R.L. Theory of Surface-Enhanced Raman Scattering in Semiconductors. J. Phys. Chem. C 2014, 118, 11120–11130.
- Hayashi, S.; Koh, R.; Ichiyama, Y.; Yamamoto, K. Evidence for Surface-Enhanced Raman Scattering on Nonmetallic Surfaces: Copper Phthalocyanine Molecules on GaP Small Particles. Phys. Rev. Lett. 1988, 60, 1085–1089.
- Xue, X.; Ji, W.; Mao, Z.; Mao, H.; Wang, Y.; Wang, X.; Ruan, W.; Zhao, B.; Lombardi, J.R. Raman Investigation of Nanosized TiO2: Effect of Crystallite Size and Quantum Confinement. J. Phys. Chem. C 2012, 116, 8792–8797.
- Pérez León, C.; Kador, L.; Peng, B.; Thelakkat, M. Characterization of the Adsorption of Ru-Bpy Dyes on Mesoporous TiO2 Films with UV-Vis, Raman, and FTIR Spectroscopies. J. Phys. Chem. B 2006, 110, 8723–8730.
- Goff, A.H.; Joiret, S.; Falaras, P.; Curie, M. Raman Resonance Effect in a Monolayer of Polypyridyl Ruthenium (II) Complex Adsorbed on Nanocrystalline TiO2 via Phosphonated Terpyridyl Ligands. J. Phys. Chem. B 1999, 103, 9569–9575.
- Shoute, L.C.T.; Loppnow, G.R. Excited-State Dynamics of Alizarin-Sensitized TiO2 Nanoparticles from Resonance Raman Spectroscopy. J. Chem. Phys. 2002, 117, 842–850.
- Blackbourn, R.L.; Johnson, C.S.; Hupp, J.T. Surface Intervalence Enhanced Raman Scattering from Fe(CN)6 on Colloidal Titanium Dioxide. A Mode-by-Mode Description of the Franck—Condon Barrier to Interfacial Charge Transfer. J. Am. Chem. Soc. 1991, 113, 1060–1062.
- Finnie, K.S.; Bartlett, J.R.; Woolfrey, J.L. Vibrational Spectroscopic Study of the Coordination of (2,2′-Bipyridyl-4,4′-Dicarboxylic Acid)Ruthenium(II) Complexes to the Surface of Nanocrystalline Titania. Langmuir 1998, 14, 2744–2749.
- Yang, L.; Jiang, X.; Ruan, W.; Zhao, B.; Xu, W.; Lombardi, J.R. Observation of Enhanced Raman Scattering for Molecules Adsorbed on TiO2 Nanoparticles: Charge-Transfer Contribution. J. Phys. Chem. C 2008, 112, 20095–20098.
- Musumeci, A.; Gosztola, D.; Schiller, T.; Dimitrijevic, N.M.; Mujica, V.; Martin, D.; Rajh, T. SERS of Semiconducting Nanoparticles (TiO2 Hybrid Composites). J. Am. Chem. Soc. 2009, 131, 6040–6041.
- Öner, I.H.; Querebillo, C.J.; David, C.; Gernert, U.; Walter, C.; Driess, M.; Leimkühler, S.; Ly, K.H.; Weidinger, I.M.; Leimk, S.; et al. Hohe Elektromagnetische Feldverstärkung in Nanotubularen TiO2-Elektroden. Angew. Chem. 2018, 130, 7344–7348.
- Maznichenko, D.; Venkatakrishnan, K.; Tan, B. Stimulating Multiple SERS Mechanisms by a Nanofibrous Three-Dimensional Network Structure of Titanium Dioxide (TiO2). J. Phys. Chem. C 2013, 117, 578–583.
- Han, X.X.; Köhler, C.; Kozuch, J.; Kuhlmann, U.; Paasche, L.; Sivanesan, A.; Weidinger, I.M.; Hildebrandt, P. Potential-Dependent Surface-Enhanced Resonance Raman Spectroscopy at Nanostructured TiO2: A Case Study on Cytochrome b5. Small 2013, 9, 4175–4181.
- Öner, I.H.; Querebillo, C.J.; David, C.; Gernert, U.; Walter, C.; Driess, M.; Leimkühler, S.; Ly, K.H.; Weidinger, I.M. High Electromagnetic Field Enhancement of TiO2 Nanotube Electrodes. Angew. Chem. Int. Ed. 2018, 57, 7225–7229.
- Querebillo, C.J.; Öner, H.I.; Hildebrandt, P.; Ly, K.H.; Weidinger, I.M. Accelerated Photo-Induced Degradation of Benzidine-p- Aminothiophenolate Immobilized at Light-Enhancing TiO2 Nanotube Electrodes. Chem. Eur. J. 2019, 25, 16048–16053.
- Zhang, X.; Lei, L.; Zhang, J.; Chen, Q.; Bao, J.; Fang, B. A Novel CdS/S-TiO2 Nanotubes Photocatalyst with High Visible Light Activity. Sep. Purif. Technol. 2009, 66, 417–421.
- Shin, S.W.; Lee, J.Y.; Ahn, K.S.; Kang, S.H.; Kim, J.H. Visible Light Absorbing TiO2 Nanotube Arrays by Sulfur Treatment for Photoelectrochemical Water Splitting. J. Phys. Chem. C 2015, 119, 13375–13383.
- Shen, J.; Meng, Y.; Xin, G. CdS/TiO2 Nanotubes Hybrid as Visible Light Driven Photocatalyst for Water Splitting. Rare Met. 2011, 30, 280–283.
- Öner, I.H.; David, C.; Querebillo, C.J.; Weidinger, I.M.; Ly, K.H. Electromagnetic Field Enhancement of Nanostructured TiN Electrodes Probed with Surface-Enhanced Raman Spectroscopy. Sensors 2022, 22, 487.
- Qi, D.; Lu, L.; Wang, L.; Zhang, J. Improved SERS Sensitivity on Plasmon-Free TiO2 Photonic Microarray by Enhancing Light-Matter Coupling. J. Am. Chem. Soc. 2014, 136, 9886–9889.
- Joannopoulos, J.D.; Pierre, R.; Villeneuve, S.F. Photonic Crystals:Putting a New Twist on Light. Nature 1997, 386, 7.
- Al-Haddad, A.; Wang, Z.; Xu, R.; Qi, H.; Vellacheri, R.; Kaiser, U.; Lei, Y. Dimensional Dependence of the Optical Absorption Band Edge of TiO2 Nanotube Arrays beyond the Quantum Effect. J. Phys. Chem. C 2015, 119, 16331–16337.
- Yip, C.T.; Huang, H.; Zhou, L.; Xie, K.; Wang, Y.; Feng, T.; Li, J.; Tam, W.Y. Direct and Seamless Coupling of TiO2 Nanotube Photonic Crystal to Dye-Sensitized Solar Cell: A Single-Step Approach. Adv. Mater. 2011, 23, 5624–5628.
- Gesesse, G.D.; Li, C.; Paineau, E.; Habibi, Y.; Remita, H.; Colbeau-Justin, C.; Ghazzal, M.N. Enhanced Photogenerated Charge Carriers and Photocatalytic Activity of Biotemplated Mesoporous TiO2 Films with a Chiral Nematic Structure. Chem. Mater. 2019, 31, 4851–4863.
- Chen, J.I.L.; Loso, E.; Ebrahim, N.; Ozin, G.A. Synergy of Slow Photon and Chemically Amplified Photochemistry in Platinum Nanocluster-Loaded Inverse Titania Opals. J. Am. Chem. Soc. 2008, 130, 5420–5421.
- Zhang, X.; John, S. Enhanced Photocatalysis by Light-Trapping Optimization in Inverse Opals. J. Mater. Chem. A 2020, 8, 18974–18986.
- Huo, J.; Yuan, C.; Wang, Y. Nanocomposites of Three-Dimensionally Ordered Porous TiO2 Decorated with Pt and Reduced Graphene Oxide for the Visible-Light Photocatalytic Degradation of Waterborne Pollutants. ACS Appl. Nano Mater. 2019, 2, 2713–2724.
- Chen, J.I.L.; Von Freymann, G.; Choi, S.Y.; Kitaev, V.; Ozin, G.A. Slow Photons in the Fast Lane in Chemistry. J. Mater. Chem. 2008, 18, 369–373.
- Sordello, F.; Duca, C.; Maurino, V.; Minero, C. Photocatalytic Metamaterials: TiO2 Inverse Opals. Chem. Commun. 2011, 47, 6147–6149.
- Rajaraman, T.S.; Parikh, S.P.; Gandhi, V.G. Black TiO2: A Review of Its Properties and Conflicting Trends. Chem. Eng. J. 2020, 389, 123918.
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, Š.; Zbořil, R.; Schmuki, P. Photocatalysis with Reduced TiO2: From Black TiO2 to Cocatalyst-Free Hydrogen Production. ACS Catal. 2019, 9, 345–364.
- Chen, X.; Liu, L.; Huang, F. Black Titanium Dioxide (TiO2) Nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885.
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750.
- Tan, H.; Zhao, Z.; Niu, M.; Mao, C.; Cao, D.; Cheng, D.; Feng, P.; Sun, Z. A Facile and Versatile Method for Preparation of Colored TiO2 with Enhanced Solar-Driven Photocatalytic Activity. Nanoscale 2014, 6, 10216–10223.
- Zhu, L.; Ma, H.; Han, H.; Fu, Y.; Ma, C.; Yu, Z.; Dong, X. Black TiO2 Nanotube Arrays Fabricated by Electrochemical Self-Doping and Their Photoelectrochemical Performance. RSC Adv. 2018, 8, 18992–19000.
- Hu, W.; Zhou, W.; Zhang, K.; Zhang, X.; Wang, L.; Jiang, B.; Tian, G.; Zhao, D.; Fu, H. Facile Strategy for Controllable Synthesis of Stable Mesoporous Black TiO2 Hollow Spheres with Efficient Solar-Driven Photocatalytic Hydrogen Evolution. J. Mater. Chem. A 2016, 4, 7495–7502.
- Zhou, W.; Li, W.; Wang, J.Q.; Qu, Y.; Yang, Y.; Xie, Y.; Zhang, K.; Wang, L.; Fu, H.; Zhao, D. Ordered Mesoporous Black TiO2 as Highly Efficient Hydrogen Evolution Photocatalyst. J. Am. Chem. Soc. 2014, 136, 9280–9283.
- Zhu, G.; Yin, H.; Yang, C.; Cui, H.; Wang, Z.; Xu, J.; Lin, T.; Huang, F. Black Titania for Superior Photocatalytic Hydrogen Production and Photoelectrochemical Water Splitting. ChemCatChem 2015, 7, 2614–2619.
- Dong, J.; Han, J.; Liu, Y.; Nakajima, A.; Matsushita, S.; Wei, S.; Gao, W. Defective Black TiO2 Synthesized via Anodization for Visible-Light Photocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 1385–1388.
- Fan, C.; Chen, C.; Wang, J.; Fu, X.; Ren, Z.; Qian, G.; Wang, Z. Black Hydroxylated Titanium Dioxide Prepared via Ultrasonication with Enhanced Photocatalytic Activity. Sci. Rep. 2015, 5, 11712.
- Islam, S.Z.; Reed, A.; Nagpure, S.; Wanninayake, N.; Browning, J.F.; Strzalka, J.; Kim, D.Y.; Rankin, S.E. Hydrogen Incorporation by Plasma Treatment Gives Mesoporous Black TiO2 Thin Films with Visible Photoelectrochemical Water Oxidation Activity. Microporous Mesoporous Mater. 2018, 261, 35–43.
- Ullattil, S.G.; Periyat, P. A “one Pot” Gel Combustion Strategy towards Ti3+ Self-Doped “Black” Anatase TiO2−x Solar Photocatalyst. J. Mater. Chem. A 2016, 4, 5854–5858.
- Sun, L.; Xie, J.; Li, Q.; Wang, F.; Xi, X.; Li, L.; Wu, J.; Shao, R.; Chen, Z. Facile Synthesis of Thin Black TiO2−x Nanosheets with Enhanced Lithium-Storage Capacity and Visible Light Photocatalytic Hydrogen Production. J. Solid State Electrochem. 2019, 23, 803–810.
- Kang, Q.; Cao, J.; Zhang, Y.; Liu, L.; Xu, H.; Ye, J. Reduced TiO2 Nanotube Arrays for Photoelectrochemical Water Splitting. J. Mater. Chem. A 2013, 1, 5766–5774.
- Zhang, M.; Pei, Q.; Chen, W.; Liu, L.; He, T.; Chen, P. Room Temperature Synthesis of Reduced TiO2 and Its Application as a Support for Catalytic Hydrogenation. RSC Adv. 2017, 7, 4306–4311.
- He, M.; Ji, J.; Liu, B.; Huang, H. Reduced TiO2 with Tunable Oxygen Vacancies for Catalytic Oxidation of Formaldehyde at Room Temperature. Appl. Surf. Sci. 2019, 473, 934–942.
- Will, J.; Wierzbicka, E.; Wu, M.; Götz, K.; Yokosawa, T.; Liu, N.; Tesler, A.B.; Stiller, M.; Unruh, T.; Altomare, M.; et al. Hydrogenated Anatase TiO2 Single Crystals: Defects Formation and Structural Changes as Microscopic Origin of Co-Catalyst Free Photocatalytic H2 evolution Activity. J. Mater. Chem. A 2021, 9, 24932–24942.
- Katal, R.; Salehi, M.; Davood Abadi Farahani, M.H.; Masudy-Panah, S.; Ong, S.L.; Hu, J. Preparation of a New Type of Black TiO2 under a Vacuum Atmosphere for Sunlight Photocatalysis. ACS Appl. Mater. Interfaces 2018, 10, 35316–35326.
- Liu, N.; Zhou, X.; Nguyen, N.T.; Peters, K.; Zoller, F.; Hwang, I.; Schneider, C.; Miehlich, M.E.; Freitag, D.; Meyer, K.; et al. Black Magic in Gray Titania: Noble-Metal-Free Photocatalytic H2 Evolution from Hydrogenated Anatase. ChemSusChem 2017, 10, 62–67.
- Zhang, Y.; Xing, Z.; Liu, X.; Li, Z.; Wu, X.; Jiang, J.; Li, M.; Zhu, Q.; Zhou, W. Ti3+ Self-Doped Blue TiO2(B) Single-Crystalline Nanorods for Efficient Solar-Driven Photocatalytic Performance. ACS Appl. Mater. Interfaces 2016, 8, 26851–26859.
- Chen, X.; Liu, L.; Liu, Z.; Marcus, M.A.; Wang, W.C.; Oyler, N.A.; Grass, M.E.; Mao, B.; Glans, P.A.; Yu, P.Y.; et al. Properties of Disorder-Engineered Black Titanium Dioxide Nanoparticles through Hydrogenation. Sci. Rep. 2013, 3, 1510.
- Yang, F.; Zhang, Z.; Li, Y.; Xiao, C.; Zhang, H.; Li, W.; Zhan, L.; Liang, G.; Chang, Y.; Ning, C.; et al. In Situ Construction of Black Titanium Oxide with a Multilevel Structure on a Titanium Alloy for Photothermal Antibacterial Therapy. ACS Biomater. Sci. Eng. 2022, 8, 2419–2427.
- Zhang, W.; Gu, J.; Li, K.; Zhao, J.; Ma, H.; Wu, C.; Zhang, C.; Xie, Y.; Yang, F.; Zheng, X. A Hydrogenated Black TiO2 Coating with Excellent Effects for Photothermal Therapy of Bone Tumor and Bone Regeneration. Mater. Sci. Eng. C 2019, 102, 458–470.
- Janczarek, M.; Endo-Kimura, M.; Wang, K.; Wei, Z.; Akanda, M.M.A.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Is Black Titania a Promising Photocatalyst? Catalysts 2022, 12, 1320.
- Zhang, M.; Wu, N.; Yang, J.; Zhang, Z. Photoelectrochemical Antibacterial Platform Based on Rationally Designed Black TiO2−x Nanowires for Efficient Inactivation against Bacteria. ACS Appl. Bio Mater. 2022, 5, 1341–1347.
- Campbell, L.; Nguyen, S.H.; Webb, H.K.; Eldridge, D.S. Photocatalytic Disinfection of S. aureus Using Black TiO2−x under Visible Light. Catal. Sci. Technol. 2022, 13, 62–71.
This entry is offline, you can click here to edit this entry!
