Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
|
Cell Biology
There are several risk factors of HCC such as viral hepatitis (B, C), cirrhosis, tobacco and alcohol use, aflatoxin-contaminated food, pesticides, diabetes, obesity, nonalcoholic fatty liver disease (NAFLD), and metabolic and genetic diseases. Diagnosis of HCC is based on different methods such as imaging ultrasonography (US), multiphasic enhanced computed tomography (CT), magnetic resonance imaging (MRI), and several diagnostic biomarkers.
- hepatocellular carcinoma
- diagnosis
- imaging methods
- molecular markers
1. Hepatocellular Carcinoma
1.1. Epidemiology of Hepatocellular Carcinoma
Approximately 690,000 cases of hepatocellular carcinoma (HCC) are diagnosed annually [1]. About 80–90% of all HCC cases are closely related to chronic liver diseases (CLD) and cirrhosis of the liver. In the past three decades, it has been shown that viral infections such as hepatitis B (HBV) and C viruses (HCV), alcoholic liver disease, as well as nonalcoholic fatty liver diseases are the major risk factors for liver cirrhosis [2]. A total of 54% and 31% of HCC cases are attributed to HBV and HCV, respectively, while 15% of HCC cases are related to other causes [3].
Age, ethnicity, gender, activity level, regional distribution, and stage of underlying liver disease all affect the global incidence of HCC differently [4,5]. Globally, cases of HCC in individuals under the age of 40 years are uncommon, while the peak occurs around the age of 70. HCC is more frequent in males than females, indicating gender predilection and the difference being much greater in high-risk areas. Two factors account for this disparity. First, men are generally more exposed to hepatitis-virus- and liver-cancer-causing substances, such as alcohol and cigarettes. Second, women’s estrogen hormone may limit interleukin-6-mediated inflammation, reducing hepatic injury and compensatory proliferation, giving the impression that they have a lower risk of developing HCC. Contrarily, testosterone in men has been shown to stimulate androgen receptor signaling, which supports the growth of HCC cells [6].
Geographically, the incidence of HCC varies widely; 30 cases per 100,000 individuals are found in Southeast Asia and Central Africa, and more than 80% of HCC patients are found in Sub-Saharan Africa and East Asia [2,4]. With an annual incidence ranging from 2% to 8%, cirrhosis caused by HCV is the main risk factor for developing HCC in Europe, Japan, Latin America, and the United States. The main risk factor in China is HBV infection [7,8].
1.2. Epidemiology of HCC in Egypt
HCC is a significant health issue in Egypt. As the sixth-largest nation in the Middle East and the Arab world, the prevalence of hepatocellular carcinoma has increased from 4% to 7.2% over the past ten years [8]. Patients with chronic liver disorders have had a striking increase in HCC. Compared to other nations on the planet, Egypt has the greatest prevalence of HCV, with 14.7% of the general population being affected. According to estimates [9], 40–50% of HCC cases are associated with HCV [10]. Because HCV infection is so common, anti-schistosomal treatment with tartar emetic injections is being considered [9,11]. However, the main geographical differences in risk factors for chronic liver diseases between Egypt and other countries include a high prevalence of HCV infection, a significant burden of nonviral liver diseases such as fatty liver disease and autoimmune liver diseases, and a relatively high prevalence of metabolic syndrome. Antiviral treatments can have a significant impact on the progression and outcome of chronic liver diseases caused by HBV and HCV. Treatment goals include the sustained suppression of viral replication, a cure for the infection (in the case of HCV), and a reduction in liver-related mortality and morbidity [12,13]. It is worth mentioning that autoimmune liver diseases such as autoimmune hepatitis (AIH) and primary biliary cholangitis (PBC) can increase the risk of HCC development, particularly in the presence of cirrhosis. Regular monitoring for HCC in these patients is important for early detection and treatment [14].
2. Diagnosis of HCC
For a correct diagnosis of HCC, serum biomarkers, one or more imaging investigations, and histology and immunostaining confirmation are all necessary [15].
2.1. Imaging Methods
2.1.1. Ultrasonography (US)
Ultrasound is the first selection method for HCC because it is low-cost, noninvasive, can be repeated every 3 to 6 months without any risk, and is well accepted by patients. The sensitivity for HCC is 58–70% and it cannot detect HCC tumors less than 1 cm in size [16].
2.1.2. Multiphasic Enhanced Computed Tomography (CT)
A computed tomography (CT) scan is a three-dimensional reconstruction technique and a public test for detecting HCC with higher sensitivity than US (53–87%) for early HCC [17].
2.1.3. Magnetic Resonance Imaging (MRI)
MRI is superior in both the identification and characterization of HCC, and it is still improving. CT and MRI both have 90% sensitivity for tumors under 2 cm. However, MRI is more sensitive than CT for HCC diagnosis (80–92%), especially for extra nodules between 1 and 2 cm in size (84% vs. 47%). Because of its limited availability and high cost, MRI is only used for characterization, diagnostic confirmation, and intrahepatic tumor staging. When patients have equivocal results with US as their first modality, CT and MRI are preferable [18]. The imaging methods used in HCC diagnostic are demonstrated in Figure 1.
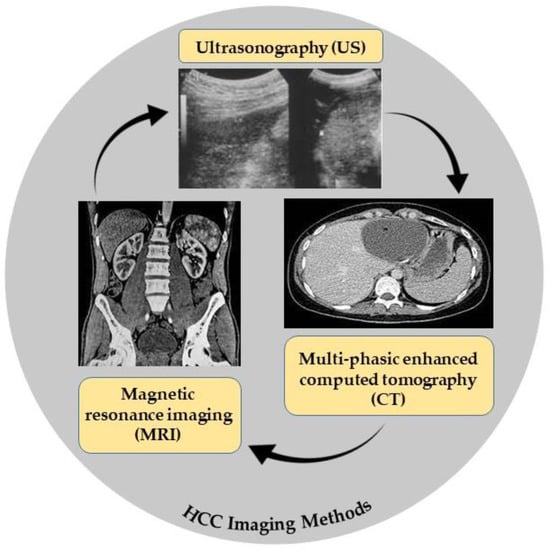
Figure 1. HCC imaging methods.
2.2. Liver Tissue Biopsy
If HCC manifests in a patient who is not cirrhotic and imaging methods are insufficient to identify HCC, liver biopsy may be required. For tumors larger than 1 cm, the American Association for the Study of Liver Diseases (AASLD) does not advise biopsy in cases when the results of two separate imaging modalities are in agreement. Liver biopsy has a sensitivity of 65–95%, absolute specificity, and PPV. Several complications are possible, such as pain, puncture of the gallbladder, bile peritonitis, and severe bleeding.
The main approach for diagnostic liver tissue assessment involves a comprehensive examination of hepatic tissue stained with hematoxylin and eosin (H&E). Additional special stains may be used to highlight or identify features that are not easily visible with H&E staining. Masson’s trichrome stain is commonly used for staining biopsy-obtained hepatic tissue, and immunostaining with polyclonal and monoclonal antibodies is also an option. However, compared to the histochemical staining methods mentioned above, immunohistochemical approaches are not frequently used in the diagnostic assessment of non-neoplastic liver diseases [19]. The diagnostic capacity of MRI was greatly enhanced to detect HCC, using several classes of contrast agents. These agents include hepatocyte-selective and reticuloendothelial-system-targeted agents such as gadobenate dimeglumine (Gd-BOPTA), mangafodipir trisodium (Mn-DPDP), and the most recently discovered hepatocyte-specific agent, gadoxetic acid (Gd-EOB-DTPA). These agents show significant paramagnetic properties to shorten liver longitudinal relaxation time, leading to enhanced sensitivity in the noninvasive detection of small hepatic lesions [20].
2.3. Liquid Biopsy
A liquid biopsy is indicated to examine tumor elements that are present in the bloodstream. Potential issues with tissue samples, noninvasiveness, and lower costs are all advantages of liquid biopsy [21]. Repeating liquid biopsies is simple throughout HCC treatment. Circulating tumor cells (CTCs), circulating cell-free DNA integrity, somatic mutations, circulating cell-free tumor DNA methylation, and circulating RNA are all examples of liquid biopsy markers [22], as shown in Figure 2A. A summary of the potential differences between tissue and liquid biopsies is shown in Figure 2B.
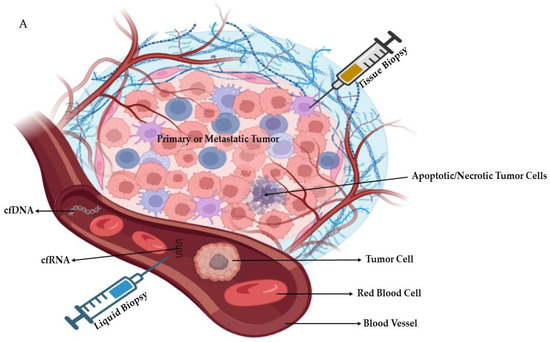
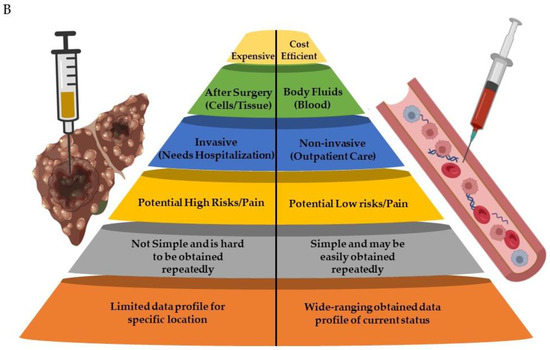
Figure 2. (A): Schematic diagram showing tissue and liquid biopsies with the examples of blood-circulating factors (cell free-RNA/-DNA, cells, etc.). (B): Comparison between liver tissue and liquid biopsies. Parts of the figure were generated using BioRender.com.
2.4. Biomarkers
A diagnostic biomarker is defined as any substance, structure, or process that can be detected, measured, or quantified in the body and aims to indicate or predict the occurrence of a disease, as well as its progression or treatment efficiency. The ideal biomarker has certain universal properties for routine clinical analysis (sensitive; specific; does not require much operator experience; inexpensive; highly reproducible; produces rapid results; correlates with tumor stages; and available samples (should be readily available like blood or urine) need no pretreatment); these are demonstrated in Figure 3 [23]. HCC marker classification according to the biochemical properties is illustrated in Figure 4.
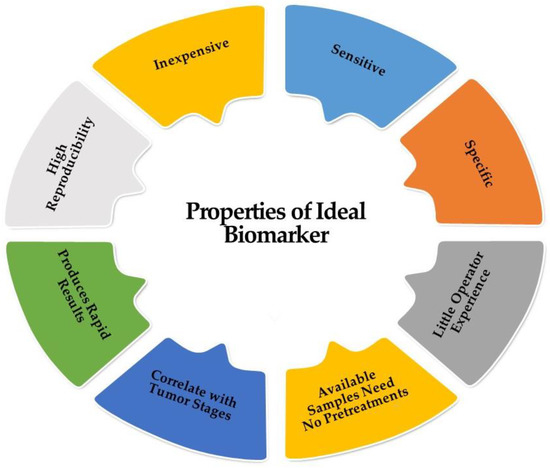
Figure 3. Characters of the ideal biomarker.
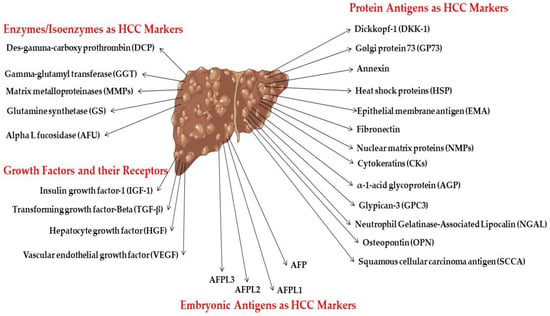
Figure 4. HCC marker classification according to biochemical nature.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11071852
This entry is offline, you can click here to edit this entry!
