Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The concept of solid matrices refers to a diverse range of waste materials generated from various sources, including agricultural, municipal, and industrial activities. These solid matrices often contain a significant amount of valuable metals, but their recovery can be challenging due to their complex composition and low metal concentration.
- biological extraction
- metals
- bioprocess
- bioleaching
- biosorption
1. Introduction
The global market size of metals and their manufactured products was valued at $11.2 trillion in 2020, and it is projected to reach $18.5 trillion by 2030 [1]. This significant growth is driven by the rising demand from diverse end-use industries, such as healthcare, aviation, energy and power, electricity, and electronics [2]. Additionally, metals play a crucial role in the construction of buildings, structures, transportation networks (including bridges and railways), as well as industries like mechanical engineering, transportation, cosmetics, catalysis, and environmental cleanup [3].
An assured supply of metals is critical to economic abundance of countries and national defense. Currently, the United States and other developed nations must import metals, causing tensions between countries partly because supply chains are diminished due to the impact of the COVID-19 pandemic [4].
The market demands metals as sustainable raw materials with high recycled content to reduce the carbon footprint of the final product [5]. Countries such as the United States, Japan, Australia, and Canada support initiatives to secure material supply through diversification, development, and recycling. The agricultural, industrial, and domestic sectors are economically interested in recovering valuable minerals, processing them, and converting them into marketable products [3].
Metal recovery through recycling refers to reprocessing waste into new metal products to reduce greenhouse gas emission levels, conserve natural resources, and manage energy consumption. In Europe, metal recycling in household solid waste is integral to sustainable waste management [6]. Countries such as Canada, the United States, and the United Kingdom, among others, promote the recycling industry with waste collection systems, separation processes, and sorting [7]. Waste management is one of the critical steps to achieving sustainable development and a circular economy [8]. Governments are tightening restrictions on waste generation and opening new landfills, putting pressure on companies to produce more environmentally friendly and sustainable goods [9].
Several metal product manufacturing companies use recyclable and recycled materials to manufacture new metal products [7]. Studies by Moreau et al. show that metals used in renewable energy technologies retain their properties and can, in principle, be recycled. This fact offers more significant potential for a circular economy [10].
It is believed that there will be the availability of material to be recycled, as the recycling market was 217.0 billion dollars in 2020 with a projection of 368.7 billion dollars by 2030 [7], added to the global generation of waste such as electronic waste with values of 42 million tons in 2014 [11], 53.6 million tons in 2019 and an estimated 74.7 million tons by 2030 [12].
Studies show that the amount of metallic fraction in unsorted mixed household waste is 5.4 kg/capita/year, which represents approximately 1.6% by weight of the total solid household waste produced. The metallic fraction of unsorted mixed household waste contains about 0.78 kg/capita/year of aluminum packaging, 2.08 kg/capita/year of metallic packaging, and 2.55 kg/capita/year of other metals. The estimated metal fraction collected from solid household waste is 1.1 kg/capita/year [6].
Recoverable material profit in e-waste was estimated at USD 57 billion in 2019 [12]. For the same year, 70.9% of steel cans were recycled along with other steel packaging types, including strapping and drums, with a 3.6% increase in steel scrap used by country and region [13].
Mixed waste is incinerated, and some metals are recovered before or after the municipal solid waste incineration process. However, the recoverability is limited since a large part of the aluminum is melted and oxidized in these, and the quality of the steel scrap is reduced [6]. In addition, when pyrometallurgical processes are used for metal recovery, organic materials, carbon, and other materials such as graphite anodes are lost after burning in the smelting furnace and cannot be recycled [14].
According to studies by Inman et al., leaching is the most common method for recovering metals from e-waste at different concentrations [4]. Leaching uses inorganic acids, including hydrochloric acid, nitric acid, sulfuric acid, sulfuric acid, phosphoric acid, and organic acids, including oxalic acid, formic acid, citric acid, tartaric acid, maleic acid, ascorbic acid, malic acid. Inorganic acids have advantages such as low cost and high leaching efficiency, although they cause equipment corrosion and secondary contamination. Leaching with organic acids can achieve the same efficiency in a milder environment, although their price is higher than that of inorganic acids, which restricts their large-scale industrial application [15].
Hydrometallurgy provides advantages for the recycling of materials, such as higher efficiency, excellent metal selectivity, lower energy consumption, and reduced emissions of hazardous gases; it faces challenges such as the production of wastewater [16] and costs due to its multiple stages in the process [14], which provides a strong motivation to study more sustainable chemistries [16].
In contrast, biohydrometallurgy brings added value to these processes, as they can recover quantities of metals using aqueous solutions and biological metabolites produced by certain microorganisms [17]; bioleaching, for example, is considered one of the green technologies for metal recovery, offering low cost in terms of installation and operation, low energy consumption, no toxic waste generation and low capital investment [1][18][19][20].
In the bioleaching process, metals are solubilized from insoluble solid substrates [1], either directly by the metabolism of microorganisms or indirectly by the products of their metabolism, such as the production of organic acids, chelating agents, amino acids, and complexing agents obtained from heterotrophic bacteria or fungi [21].
Bioleaching has received attention in a variety of industrial areas, especially in mineral and solid industrial waste materials (e.g., galvanic sludge, sewage sludge, fly ash, electronic waste, spent petrochemical catalysts, medical waste, spent batteries, residual slag), where the concentration of metals is low, where the presence of certain elements would result in smelter damage, or where environmental considerations favor biological treatment options. This process also allows the recovery of metals from low-grade sulfide ores and concentrates that cannot be economically processed by conventional techniques, as well as the production of concentrated solutions of metal salts, which could be recycled [18].
2. Recovery of Metals of Interest from Solid Matrices
The concept of solid matrices refers to a diverse range of waste materials generated from various sources, including agricultural, municipal, and industrial activities. These solid matrices often contain a significant amount of valuable metals, but their recovery can be challenging due to their complex composition and low metal concentration.
Biotechnology is a promising alternative to current industrially available technologies. One of the emerging technologies is the bioprocessing of waste materials to recover metals [11].
The recovery processes of metals of interest by biological methods involve three stages: the first is the extraction of the solute from the solvent using bioleaching, the second is the concentration process using biosorption/desorption, and the third is the deposition of the metal as a species in concentrated solution as a precipitated solid phase. Figure 1 shows a block diagram summarizing the process.
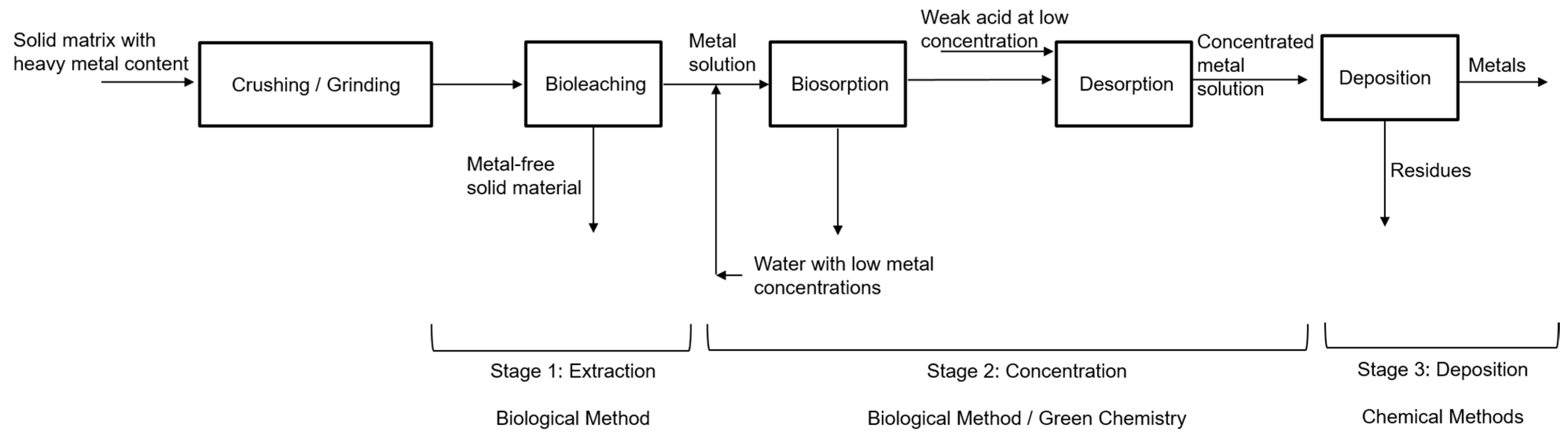
Figure 1. Recovery processes of metals of interest. Source: Authors.
Bioleaching is the first stage where the metals of interest, such as Au, Ag, As, Co, Cu, Mn, Mo, Ni, U, V and W, and Zn, among others, are extracted from primary ores and solid matrices in general. The growing academic and commercial interest in the bioprocess is attributed to its better environmental profile, ease, and practicality of operation, better profitability, the potential for future development, and excellent selectivity with metals. Metals are extracted from solid matrices even in low concentrations [11]. In this bioprocess, solid matrices with metal content (SMMC) such as mining waste (tailings—low-grade ore), consumer waste such as electronic scrap, used batteries, municipal solid waste, and agricultural waste are used as raw materials, moving towards a circular economy, which is considered a green alternative with lower energy costs and environmental impacts compared to traditional metallurgical processes [12].
The second phase is the concentration of metals in the solution, where the biosorption/desorption process is employed. Biosorption is carried out in continuous systems in packed bed columns with microorganisms immobilized in porous matrices, as shown in the study by Ramírez et al. [22]. The use of residual yeast or fungi from the [23] industry is recommended, especially from the brewing industry, due to their volume of generation, easy acquisition, and low cost.
After the adsorption process, desorption is carried out; in this process, the aim is to separate and recover the metals retained in the packed bed column so that a concentrated solution of these metals is generated. For the desorption process, various extracting solutions or eluting agents are used, such as chelating agents (EDTA), acidic solutions (HCl, H2SO4, HNO3), inorganic and organic salts (NaNO3, Ca(NO3)2, sodium citrate), among others [24][25]. This process protonates the active sites of the microorganisms immobilized in the column, activating them again [25][26], which makes the development of the following adsorption process more efficient and allows the reuse of the packed column [24][27].
In the third phase, the metal as a concentrated solution species is deposited as a stable solid phase using conventional chemical methods such as precipitation and electrodeposition or reduction processes [12], which employ reducing agents that lower the redox potential such as SnCl2, FeSO4, SO2. Recently, organic reducing agents such as thiourea, glucose, sucrose, lactose [28], and even cellulose, hemicellulose, and lignin present in different biomasses are being explored, which are “greener” due to their low toxicity and produce less harmful gas emissions compared to inorganic reductants [29][30].
This entry is adapted from the peer-reviewed paper 10.3390/su151310222
References
- Pourhossein, F.; Mousavi, S.M. A Novel Rapid and Selective Microbially Thiosulfate Bioleaching of Precious Metals from Discarded Telecommunication Printed Circuited Boards (TPCBs). Resour. Conserv. Recycl. 2022, 187, 106599.
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of Lithium and Cobalt from Spent Lithium Ion Batteries (LIBs) Using Organic Acids as Leaching Reagents: A Review. Resour. Conserv. Recycl. 2018, 136, 418–435.
- Yadav, V.K.; Yadav, K.K.; Tirth, V.; Gnanamoorthy, G.; Gupta, N.; Algahtani, A.; Islam, S.; Choudhary, N.; Modi, S.; Jeon, B. Extraction of Value-Added Minerals from Various Agricultural, Industrial and Domestic Wastes. Materials 2021, 14, 6333.
- Inman, G.; Nlebedim, I.C.; Prodius, D. Application of Ionic Liquids for the Recycling and Recovery of Technologically Critical and Valuable Metals. Energies 2022, 15, 628.
- Steinbach, V.; Wellmer, F.-W. Consumption and Use of Non-Renewable Mineral and Energy Raw Materials from an Economic Geology Point of View. Sustainability 2010, 2, 1408–1430.
- Kuusiola, T.; Wierink, M.; Heiskanen, K. Comparison of Collection Schemes of Municipal Solid Waste Metallic Fraction: The Impacts on Global Warming Potential for the Case of the Helsinki Metropolitan Area, Finland. Sustainability 2012, 4, 2586–2610.
- Business Wire the Worldwide Metal Recycling Industry Is Expected to Reach $368.7 Billion by 2030. Available online: https://www.businesswire.com/news/home/20220503005770/en/The-Worldwide-Metal-Recycling-Industry-is-Expected-to-Reach-368.7-Billion-by-2030---ResearchAndMarkets.com#:~:text=The%20global%20metal%20recycling%20market,5.2%25%20from%202021%20to%202030 (accessed on 28 February 2023).
- Psomopoulos, C.S.; Kungolos, A.; Di Nardo, A. Advances in Industrial Waste Reduction. Appl. Sci. 2023, 13, 1403.
- De Colle, M.; Puthucode, R.; Karasev, A.; Jönsson, P.G. A Study of Treatment of Industrial Acidic Wastewaters with Stainless Steel Slags Using Pilot Trials. Materials 2021, 14, 4806.
- Moreau, V.; Dos Reis, P.; Vuille, F. Enough Metals? Resource Constraints to Supply a Fully Renewable Energy System. Resources 2019, 8, 29.
- Işıldar, A.; van de Vossenberg, J.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N.L. Biorecovery of Metals from Electronic Waste. In Sustainable Heavy Metal Remediation; Case Studies; Spring: Berlin/Heidelberg, Germany, 2017; Volume 2, pp. 241–278.
- Van Yken, J.; Boxall, N.J.; Cheng, K.Y.; Nikoloski, A.N.; Moheimani, N.R.; Kaksonen, A.H. E-Waste Recycling and Resource Recovery: A Review on Technologies, Barriers and Enablers with a Focus on Oceania. Metals 2021, 11, 1313.
- The Business Research Company Metal Products Global Market Opportunities & Strategies. Available online: https://www.thebusinessresearchcompany.com/report/metal-products-global-market (accessed on 1 March 2023).
- Guo, H.; Min, Z.; Hao, Y.; Wang, X.; Fan, J.; Shi, P.; Min, Y.; Xu, Q. Sustainable Recycling of LiCoO2 Cathode Scrap on the Basis of Successive Peroxymonosulfate Activation and Recovery of Valuable Metals. Sci. Total Environ. 2021, 759, 143478.
- Liu, M.; Ma, W.; Zhang, X.; Liang, Z.; Zhao, Q. Recycling Lithium and Cobalt from LIBs Using Microwave-Assisted Deep Eutectic Solvent Leaching Technology at Low-Temperature. Mater. Chem. Phys. 2022, 289, 126466.
- Ma, C.; Svärd, M.; Forsberg, K. Recycling Cathode Material LiCo1/3Ni1/3Mn1/3O2 by Leaching with a Deep Eutectic Solvent and Metal Recovery with Antisolvent Crystallization. Resour. Conserv. Recycl. 2022, 186, 106579.
- Blázquez Izquierdo, M.L.; Muñoz Sánchez, J.A.; Castro, L. Special Issue “Leaching/Bioleaching and Recovery of Metals”. Available online: https://www.mdpi.com/journal/metals/special_issues/leaching_bio_recovery (accessed on 19 January 2023).
- Mahmoud, A.; Cézac, P.; Hoadley, A.F.A.; Contamine, F.; D’Hugues, P. A Review of Sulfide Minerals Microbially Assisted Leaching in Stirred Tank Reactors. Int. Biodeterior. Biodegrad. 2017, 119, 118–146.
- Deng, X.; Chai, L.; Yang, Z.; Tang, C.; Wang, Y.; Shi, Y. Bioleaching Mechanism of Heavy Metals in the Mixture of Contaminated Soil and Slag by Using Indigenous Penicillium Chrysogenum Strain F1. J. Hazard. Mater. 2013, 248–249, 107–114.
- Khodadadmahmoudi, G.; Abdollahi, H.; Mohammadzadeh, A.; Saneie, R.; Mirmohammadi, M.; Rezaei, A.; Jozanikohan, G.; Naderi, H. Green Extraction of Nickel and Valuable Metals from Pyrrhotite Samples with Different Crystallographic Structures through Acidophilic Bioleaching. J. Environ. Manag. 2022, 317, 115394.
- Dusengemungu, L.; Kasali, G.; Gwanama, C.; Mubemba, B. Overview of Fungal Bioleaching of Metals. Environ. Adv. 2021, 5, 100083.
- Ramírez Carmona, M.E.; Pereira da Silva, M.A.; Ferreira Leite, S.G.; Vasco Echeverri, O.H.; Ocampo-López, C. Packed Bed Redistribution System for Cr(III) and Cr(VI) Biosorption by Saccharomyces Cerevisiae. J. Taiwan Inst. Chem. Eng. 2012, 43, 428–432.
- Isaza-Pérez, F.; Ramírez-Carmona, M.; Rendón-Castrillón, L.; Ocampo-López, C. Potential of Residual Fungal Biomass: A Review. Environ. Sci. Pollut. Res. 2020, 27, 13019–13031.
- Gómez-Aguilar, D.L.; Esteban-Muñoz, J.A.; Rodríguez-Miranda, J.P.; Baracaldo-Guzmán, D.; Salcedo-Parra, O.J. Desorption of Coffee Pulp Used as an Adsorbent Material for Cr(III and VI) Ions in Synthetic Wastewater: A Preliminary Study. Molecules 2022, 27, 2170.
- Lodeiro, P.; Herrero, R.; Sastre de Vicente, M.E. Batch Desorption Studies and Multiple Sorption–Regeneration Cycles in a Fixed-Bed Column for Cd(II) Elimination by Protonated Sargassum Muticum. J. Hazard. Mater. 2006, 137, 1649–1655.
- Renu; Agarwal, M.; Singh, K. Heavy Metal Removal from Wastewater Using Various Adsorbents: A Review. J. Water Reuse Desalination 2017, 7, 387–419.
- Ramírez-Carmona, M.; Ocampo-López, C.; Rendón-Castrillon, L.; Vélez-Salazar, Y.; Muñoz-Blandón, O.; Salazar-Martínez, S. System for Separating Fluids and Method for the Application Thereof. 2015, pp. 1–17. Available online: https://www.freepatentsonline.com/WO2015011672.pdf (accessed on 28 February 2023).
- Benavente, O.; Hernández, M.C.; Melo, E.; Núñez, D.; Quezada, V.; Zepeda, Y. Copper Dissolution from Black Copper Ore under Oxidizing and Reducing Conditions. Metals 2019, 9, 799.
- Sinha, M.K.; Purcell, W. Reducing Agents in the Leaching of Manganese Ores: A Comprehensive Review. Hydrometallurgy 2019, 187, 168–186.
- Vélez-Salazar, Y. Desarrollo de un Sistema de Recuperación de Oro Proveniente de la Lixiviación con Caldos Fermentados de Origen Fúngico a Través de un Proceso Carbon-in-Leach. Ph.D. Thesis, Universidad Pontificia Bolivariana, Medellín, CO, USA, 2020.
This entry is offline, you can click here to edit this entry!
