1. Enzymatic Collagen Cross-Linking Mediated by Lysyl Oxidases
It has been well-established that lysyl oxidase acts upon its substrate’s collagen, elastin and nonpeptidyl amines, following a ping-pong mechanism [
45,
46,
47]. For collagens, after the secretion of procollagen molecules outside of the cell, lysyl oxidase catalyzes the conversion of lysine and hydroxylysine residues into aldehydes in nonhelical telopeptide regions. Aldehydes react with lysine or hydroxylysine residues contained in the triple-helical domain on an adjacent collagen molecule to form immature divalent cross-links that can also spontaneously react with another divalent cross-links to create mature trivalent cross-links [
47]. Furthermore, it has been observed that when the lysine-derived cross-links are formed by hydroxylysine-derived aldehydes, they are more stable than those formed from the lysine aldehyde pathway [
5].
LOX enzymes regulate several biological processes, including extracellular matrix stabilization, cellular growth, and homeostasis [
48,
49]. Still, the primary role of this enzyme family is to participate in the remodelling of extracellular matrices through the formation of inter- and intrachain cross-links in collagen and elastin. Primarily, LOX enzymes promote the first step in the formation of covalent cross-linking to stabilize collagen fibrils [
12,
50,
51]. Specifically, for fibrillar type I collagen, it has been reported that LOX and LOXL2 can form covalent cross-links in the molecule [
52]. It has also been demonstrated that LOX, LOXL2 and LOXL4 cross-link collagen IV, along with indications that LOXL4 cross-links IV via an increase in collagen IV deposition in vascular matrix remodeling [
47,
53,
54,
55].
It has been shown that LOX activity is vital for wound-healing but also involved in the pathogenesis of fibrotic diseases; more precisely, its dysregulated activity promotes ineffective or excessive collagen cross-linking, which drives multiple diseases [
49,
56]. In this context, the formation of collagen cross-linking has been associated with many chronic diseases, including diabetes, cancer metastasis, osteoarthritis and vascular and fibrotic diseases [
57,
58,
59]. In addition, the augmented cross-linking activity of these enzymes is responsible for large insoluble extracellular proteins that are resistant to proteolysis reported in several pathological conditions. It has also been associated with the increased deposition of fibrillar collagens in fibrotic areas. The dysregulation of expression and activity of lysyl oxidases have been found to correlate with numerous diseases and adverse physiological states, including fibrosis in different organs such as the liver, lung, and kidney [
60,
61,
62]. Very recently, it has been proposed that LOXL4 in the main LOX activity and is a critical determinant of collagen cross-linking in lung fibrosis [
63]. Among all lysyl oxidases enzymes, the isoforms LOX and LOXL2 are widely associated with metastasis progression because they are needed in the production of a permissive niche to maintain metastatic tumor cell growth [
64]. Lastly, it has been proposed that defining tissue-specific variance in collagen cross-linking may help to create biomarkers of pathological connective tissues [
13].
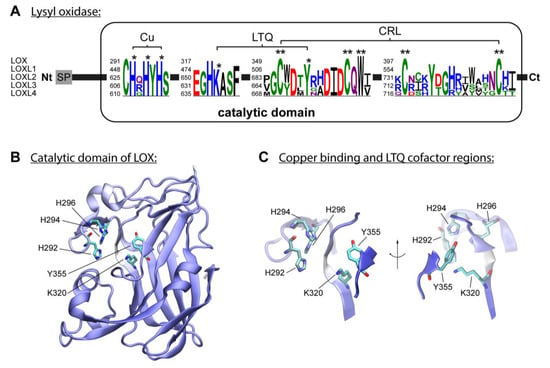
Five members constitute the mammal LOX, which are classified according to primary structure and functions: LOX, LOXL1, LOXL2, LOXL3 and LOXL4 [
12]. Members were divided into two subfamilies based on a phylogenetic study described by the Rodriguez-Pascual group [
65]. The first subfamily includes LOX and LOXL1, and the second comprises LOXL2, LOXL3 and LOXL4. These enzymes are classified as copper amine oxidases and display a conserved catalytic domain that contains the copper-binding site [
47]. The enzymatic reaction of lysyl oxidases isoforms requires, besides copper, the organic quinone cofactor named lysyl tyrosylquinone (LTQ) (
Figure 1A) [
66,
67]. The copper ion is incorporated into LOX in the trans-Golgi network by ATP7A, a copper-transporting P-type ATPase 1 [
47,
68]. Three histidine residues, H292, H294 and H296, coordinate the essential copper cation in LOX [
47,
69]. In LOXL2, the equivalent histidine residues H662, H628, and H630 form the copper-binding site [
70,
71]. The LTQ is formed by specific residues within the nascent enzyme, derived from tyrosine Y355 and lysine K320 in the LOX isoform. For LOXL2, Mure and colleagues described the spatial arrangement of LTQ between Y689 and K653 and their position relative to the coordination site of Cu
2+ [
66,
70,
72]. The N-terminal domain differs to the highly conserved catalytic domain: AlphaFold provides a protein structure of hLOX (Uniprot-ID: P28300) [
73,
74] (
Figure 1B,C). The catalytic domain is modelled with quality attributes of confidence and very high confidence according to AlphaFold’s confidence score (blue-colored regions in the protein structure). Only the area around K320 shows low confidence (white-colored region in the protein structure). This is likely due to the flexibility of this region, which allows for conformational adjustments during LTQ co-factor formation, as proposed by Meier and colleagues [
70].
Figure 1. The lysyl oxidase family. (A) LOX isoforms contain a signal peptide (SP) in the amino-terminal region and a catalytic domain in the highly conserved carboxyl-terminal region. The catalytic domain includes a copper-binding domain (Cu), lysyl tyrosylquinone cofactor (LTQ) and cytokine receptor-like domain (CRL), which are also present in isoforms LOXL1, LOXL2, LOXL3 and LOXL4. Three histidine residues forming the copper-binding site are conserved in human LOX isoforms. Respective histidine residues are marked with an asterisk in the sequence logos based on the five human LOX isoforms. The conserved lysine y tyrosine residues forming the LTQ are also marked with an asterisk in the sequence. Conserved residues of the CRL domain are marked with two asterisks. (B) AlphaFold model of hLOX lysyl oxidase-like domain represented in NewCartoon. The color illustrates the confidence of the model structure, with white indicating low-confidence regions and blue high-confidence regions. Residues H292, H294 and H296, and LTQ cofactor residues K320 and Y355, which form copper-binding sites, are highlighted. (C) Detailed view of the residues highlighted in (B). Note that the confidence of the structure around residue K320 is low, which indicates uncertainty in this region (see main text for more information).
On the other hand, the N-terminal domain of the LOX protein differs from the highly conserved catalytic domain. LOX and LOXL1 contain a propeptide region in their N-terminal part, whereas LOXL2, LOXL3 and LOXL4 contain four scavenger receptor cysteine-rich domains (SRCR) [
12]. This type of domain has been involved in the proteolytic processing of the LOXL2 isoform by the proprotein convertase PACE4 [
47,
75]. Two N-glycosylation sites have been described in LOXL2 that are present in the second and fourth SRCR domains [
12,
76]. There are important differences in the modulation of catalytic activity of LOX isoforms and their molecular mechanisms [
75].
It has been proposed that the formation of immature enzymatic cross-links, generated during collagen fibrillogenesis by the enzymatic activity of lysyl oxidase, is a beneficial process in development [
2]. In contrast, the formation of mature cross-links damages connective tissues over time and is particularly associated with ageing [
39]. Examples of immature reducible and divalent cross-links are the aldimine and keto-imine bonds that form in newly synthesized collagens [
7,
41]. In particular, aldimine bonds are formed between an aldehyde and an amine group through a condensation reaction, where the carbonyl group of the aldehyde reacts with the amine group to form a Schiff base [
1,
77]. The resulting molecule is named an aldimine- or imine-containing cross-link (
Table 1). It has been established that dehydro-lysinonorleucine (deH-LNL), dehydro-hydroxylysinonorleucine (deH-HLNL) and dehydro-dihydroxylysinonorleucine (deH-DHLNL) are immature divalent cross-links that have shown to be crucial to the formation of more complex cross-links in type I collagen. Ketoimine cross-links are another type of immature divalent cross-links when lysine-keto-norleucine (LKNL) and hydroxylysine-keto-norleucine (HLKNL) are produced via Amadori rearrangements using deH-HLNL and deH-DHLNL cross-links [
1] (
Table 1). These bifunctional reducible cross-links undergo spontaneous maturation into nonreducible trivalent cross-links, such as pyridinoline and deoxypyridinoline (found in bone and cartilage), pyrrole cross-links (present in bone), arginoline (found in cartilage), and histidinohydroxylysinonorleucine (found in skin) [
78,
79]. The presence of these specific cross-links highlights their tissue specificity [
7]. Interestingly, cross-links associated with histidine were reported to be artefacts found in mass spectrometry [
80]. However, this type of cross-linking has been detected in vivo, suggesting that it can be susceptible to the low pH that produces cross-link degradation [
1].
When compared to the effects of cross-linking elastin, the repercussions of collagen cross-linking are very different. This is primarily the result of the precise packing of the collagen polypeptide chains into a rigid triple-helix and the self-assembly of these molecules into fibrils, which limits the number of residues that are accessible to lysyl oxidase [
81]. Secondly, the presence of hydroxylysine in collagen modifies the subsequent reactions of the initial cross-links. These two factors work in tandem to produce this effect. The lysine or hydroxylysine residue that is present in the short non-helical N- and C-terminal portions of the molecule is oxidized after the lysyl oxidase binds to the freshly formed fibril and oxidizes it. Because of the end-overlap packing, the helical portions of one molecule, which is opposite the nonhelical terminal region of a neighboring molecule, are where the enzyme attaches itself to the fibril [
7]. The enzyme does not act on the individual molecules; it only acts on the fibril.
Proteins with a collagen-like domain include complement C1q, mannose-binding protein C, pulmonary-surfactant-associated proteins A1, A2, and D, and gliomedin. Some of them are known as soluble defensive collagens because they have a recognition domain that is contiguous with a collagen-like triple-helical domain [
7,
82], while gliomedin is known as a membrane collagen [
83]. Due to the lack of hydroxylysine residues and the structural constraint for lysyl oxidases to build such links, which require action on the produced fibril, it is likely that such cross-linking does not occur in collagen-like proteins.
Significantly, aging often manifests in two contrasting scenarios: an excessive local deposition of collagen, as seen in fibrosis, or a gradual overall reduction in collagen mass [
84]. As individuals age, the normal cross-linking of collagen in connective tissues diminishes due to the cumulative damage from collagen fragmentation, oxidation, and glycation [
85]. This progressive decline in collagen mass, observed not only in supporting tissues but also in other organs, compromises the integrity of the extracellular matrix and has implications for age-related conditions like diabetes, cancer, chronic liver disease, and cardiovascular diseases [
86]. Simultaneously, the accumulation of molecular damage, chronic inflammation, or injury during aging can drive abnormal collagen deposition, leading to fibrosis [
37,
87]. Furthermore, the deficiency of the LOX enzyme in adult skin has been associated with inadequate or abnormal collagen cross-linking, which contributes to skin aging [
88]. These observations indicate a general decline in enzymatic collagen cross-linking with age. However, due to the increased occurrences of damage or injury, atypical collagen accumulation, as observed in fibrosis, can transpire.
2. Lysyl Oxidase Activity of Polyphenols
Polyphenols, which are derived from plants, fruits, vegetables, floral tissues, stems, bark, and roots, serve as major sources of polyphenolic compounds, and are the most varied group of phytochemicals [
23]. Extensive research has been conducted on these natural products due to their well-known health benefits and protective effects [
16,
23]. Among the polyphenols, flavonoids play a crucial role and are associated with various positive health outcomes, including increased longevity and reduced risk of cardiovascular diseases in populations with a diet rich in flavonoids [
24,
113]. These beneficial effects are primarily attributed to the potent antioxidant activity exhibited by polyphenols. However, it is important to note that, under certain conditions, polyphenols can also act as prooxidants, promoting the oxidation of other compounds [
20,
21,
22]. This prooxidant activity may be linked to the non-enzymatic, metal-catalyzed oxidation of polyphenols, leading to the generation of hydrogen peroxide (H
2O
2). This non-enzymatic system can lead oxidative modification of proteins by oxidative deamination of the ε-amine group of lysine to α-aminoadipic-5-semialdehyde, the main carbonyl product, similar to the reaction of LOX enzymes [
20,
21]. Therefore, the biological activities of polyphenols, including their behavior as antioxidants or prooxidants, are believed to be concentration-dependent and are directly proportional to the total number of hydroxyl groups, especially those present in the B-ring of flavonoid molecules [
24].
Several studies have described the properties of specific flavonoids in preserving collagen stability [
114,
115]. For instance, anthocyanidins, natural plant pigments found in fruits, flowers, and certain vegetables, have been shown to stabilize collagens [
116,
117]. Extracts rich in anthocyanidins inhibit collagen degradation, reduce metalloprotease (MMP) activity, and protect against UV radiation in dermal fibroblast models, thus preventing UV-induced skin photoaging [
118]. Furthermore, flavonoids can stimulate the production of fibrillar collagen in mouse fibroblast models. Another mechanism associated with collagen stability preservation involves the inhibition of collagenase and elastase activity [
119].
Importantly, polyphenols and flavonoids have been found to contribute to the formation of non-covalent and covalent collagen cross-links [
1,
114]. It has been established that polyphenols possess amine oxidase-like activity in the presence of Cu
2+, which is essential for facilitating appropriate collagen and elastin cross-linking [
20]. Several polyphenols from different plant species, including chlorogenic acid, gallic acid and caffeic acid, have been associated with this amine oxidase-like activity [
21]. It has been assumed that these polyphenols are converted to the o-quinones and acquire a lysyl oxidase-like activity [
21,
24]. In addition, the conversion of catechol-type polyphenols into o-quinone derivatives has also been observed [
20]. These quinones can catalyze the oxidative deamination of primary amines by polyphenols, leading to the formation of iminoquinone and iminophenol, and ultimately resulting in the oxidation product α-aminoadipic-5-semialdehyde [
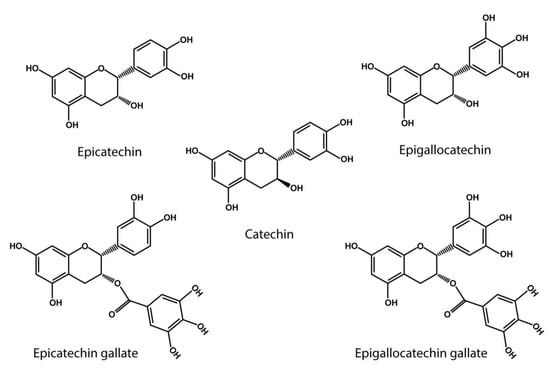
20]. Notably, the catechol-type polyphenols, which includes catechin (C), epicatechin (EC), epigallocatechin (EGC), epigallocatechin gallate (EGCG), gallocatechin gallate (GCG), and epicatechin gallate (ECG), are flavonoids primarily found in green tea and grapes [
16,
22] (
Figure 2). In particular EGCG has shown a high capacity to oxidize specific lysine residues, further contributing to oxidative deamination [
20]. The analog of resveratrol, piceatannol, has been associated with the formation of dehydrolysinonorleucine (HLNL) [
120].
Figure 2. Catechol- type polyphenol structures: Chemical structures of catechin derivatives, which are proposed as putative quinone cofactors due to their amine oxidase-like activity, which could be attributed to polyphenols, or where these polyphenols could be required. Catechin (C), epicatechin (EC), epigallocatechin (EGC), epigallocatechin (EGCG), gallocatechin gallate (GCG), epicatechin gallate (ECG).
It has been established that the failure to form covalent cross-links results in the increased degradation of collagen molecules [
40,
121]. Considering the impact of both polyphenol activities on collagen cross-linking, it is interesting to explore their influence on the regulation of fibrotic processes and wound healing. However, further studies are necessary to elucidate the effect of natural extracts on collagen cross-linking. Most scientific investigations have primarily focused on evaluating the biosynthesis and deposition of the extracellular matrix. Therefore, it is crucial to examine the levels of cross-linking and the molecular mechanisms involved in the interaction between flavonoids and lysyl oxidase activity. This will enable us to establish appropriate methods for accurately measuring collagen cross-linking. Additional research is essential to enhance our understanding of the previously undefined roles of natural extracts and their beneficial effects on collagen biosynthesis and enzymatic cross-linking.
3. Anti-Glycating Activity of Polyphenols
At present, a compelling body of evidence supports the anti-glycation activities of polyphenols. Glycation is a spontaneous and non-enzymatic reaction between reducing sugars, such as glucose and fructose, and free amino groups of proteins, DNA, and lipids, which render an unstable Schiff base and then convert it to more stable structures known as Amadori products. These products may undergo a complex series of reactions, leading to the formation of advanced glycation end-products (AGEs). The formation of AGEs occurs at a very high rate in the presence of hyperglycemia and tissue oxidative stress [
102].
The pathological implications of AGEs formation are extensively supported in many human diseases. Although initially ascribed to the mechanisms underlying the micro- and macrovascular complications in Diabetes Mellitus [
105,
122], the contribution of advanced glycation end-products to many human diseases is well-documented [
101,
123]. The pathologic effects of AGEs are mainly related to their ability to promote oxidative stress and inflammation by binding to the receptor for advanced glycation end-products (RAGE) [
124], or by cross-linking with proteins and thus altering their structure and function [
125]. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis.
Polyphenols can significantly reduce the unhealthy consequences of advanced glycation end-products by different mechanisms. These effects are achieved by interfering with either RAGE expression and signaling, or by inhibiting the cross-linking with body proteins [
28]. Notably, the trapping capacity of dicarbonyls compounds, particularly methylglyoxal (MGO) or glyoxal (GO), has been reported for some polyphenols, and deserves special attention because these dicarbonyls are considered one of the most efficient protein crosslinkers [
103].
In this regard, several polyphenols displayed important dicarbonyls-trapping activities, as reported for quercetin [
126], chrysin derivatives [
127], genistein [
128], epigallocatechin-3-gallate [
129], as well as for resveratrol and different hydroxycinnamic acids [
130,
131] among many other polyphenols. Of note, this activity has been reported not only for soluble polyphenols but also for bound-polyphenol-rich insoluble dietary fiber [
132].
This entry is adapted from the peer-reviewed paper 10.3390/ijms241310985