Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Heart failure with preserved ejection fraction (HFpEF) is a heterogeneous clinical syndrome with multiple underlying mechanisms and comorbidities that leads to a variety of clinical phenotypes. The identification and characterization of these phenotypes are essential for better understanding the precise pathophysiology of HFpEF, identifying appropriate treatment strategies, and improving patient outcomes.
- heart failure with preserved ejection fraction
- artificial intelligence
- cluster
1. Introduction
Heart failure with preserved ejection fraction (HFpEF) is diagnosed as heart failure with a left ventricular ejection fraction (LVEF) of ≥50% and elevated left ventricular filling pressures at rest or during exercise after careful exclusion of conditions that may mimic HFpEF [1,2]. HFpEF is a complex clinical syndrome that differs from other cardiovascular diseases, as it is defined by a combination of symptoms, signs, and other manifestations rather than a specific diagnostic test.
There is currently little evidence supporting the effectiveness of conventional therapies utilized for HFpEF to reduce mortality rates, such as empagliflozin in the EMPEROR-Preserved trial and dapagliflozin in the DELIVER trial. However, emerging research suggests that treatment should be tailored to the specific comorbidities present in each patient [3]. Some of the most common comorbidities seen in patients with heart failure include coronary artery disease, atrial fibrillation (AF), obesity, diabetes, renal impairment, and pulmonary hypertension. Accordingly, HFpEF can be classified into different phenotypes based on various criteria, including underlying etiology, clinical characteristics, and comorbidities [3,4,5,6,7,8]. Detailed molecular signaling, gene ontology functional analysis, and the use of the Kyoto Encyclopedia of Genes and Genomes pathway also potentiate the precise mechanisms of action and targets of SGLT2 inhibitors in clinical practice [9,10].
2. Management of HFpEF Phenotype Based on “SwedeHF” and “CHECK-HF” Registries
Personalized management of different HFpEF phenotypes using clustering targeting more specific molecular or pathological etiology driving underlying mechanisms has been proposed in several studies [27,40,41,42]. For example, obesity-related HFpEF with or without hyperlipidemia or diabetes may benefit from combined sodium–glucose cotransporter-2 inhibitors (SGLT2i), mineralocorticoid receptor antagonists (MRA), and angiotensin receptors/neprilysin inhibitor (ARNi) due to an inner deficiency of effective natriuretic peptide from excessive visceral adiposity [43].
2.1. Cluster 1
Among the five clusters, patients with HFpEF in this group had a median age of 59 years and a relatively low burden of comorbidities, making them the youngest of the cohorts. The most common comorbidities in Cluster 1 were hypertension (46%) and obesity (42%). The principles of management for this group are to control blood pressure and reduce body weight. It is worth mentioning that cluster 1 includes patients who have recovered HFrEF, due to the higher percentage of implantable cardioverter-defibrillator or cardiac resynchronization therapy.
In addition to the implantable devices, quite a few medications have demonstrated an established efficacy in previous HFpEF trials. Those drugs were renin–angiotensin–aldosterone system (RAAS) antagonists such as angioten-sin-converting enzyme inhibitors (ACEis), angiotensin II receptor blockers (ARBs), mineralocorticoid receptor antagonists (MRAs), and angiotensin receptors/neprilysin inhibitor (ARNi), which could be considered as first-line agents for the management of HFpEF. Lifestyle modifications were strongly suggested for this cluster. Significant improvements in quality of life and exercise tolerance were observed as a result of weight reduction, which was found to be safe. In addition to these benefits, weight loss in patients with HFpEF has been shown to have a positive impact on cardiac function and metabolic parameters, potentially leading to reduced doses of diuretics, antihypertensive agents, and diabetes medications.
2.2. Cluster 2
The individuals belonging to Cluster 2 were relatively older compared to those in Cluster 1, having a median age of 77 years. This cluster included patients with HFpEF characterized by AF without diabetes. Principles of management for this cluster align with the AF Better Care (ABC) pathway, including rate/rhythm control in AF management, as follows: (A) avoiding thromboembolic events with the use of anticoagulation. (B) better management of symptoms with personalized, symptom-directed decisions on rate or rhythm control. Rate control involves the use of beta-blockers/non-dihydropyridine (DHP) calcium channel blockers (CCBs) (diltiazem or verapamil)/digoxin; rhythm control involves the use of amiodarone/dronedarone or AF ablation. (C) Effective management of cardiovascular and coexisting conditions, including attention to psychological factors and lifestyle. Following the ABC pathway has been shown to lead to improved outcomes, including decreased risks of all-cause mortality, cardiovascular mortality, stroke, and hospitalization due to cardiovascular reasons. It is important to avoid excessive rate control in patients with both HFpEF and AF, as it may diminish their chronotropic reserve. In a trial comparing strict (<80 bpm) and lenient (<110 bpm) rate control in patients with AF, which may have included individuals with undiagnosed HFpEF, no significant differences in outcomes were observed.
2.3. Cluster 3
Among the five clusters, Cluster 3 patients were the oldest (median age, 88 years) with the highest N-terminal pro b-type natriuretic peptide (NT-proBNP) values. It was reasonable to eliminate any meaningful clinical phenotyping for this cluster, as it presented with an anticipated higher risk for an ominous outcome. Clinically, the principle of for the elderly is to reduce hospitalization rates and improve quality of life. Decongestion of diuretics has been shown to reduce hospitalization rates. In the TOPCAT trial, spironolactone was associated with a decrease in heart failure hospitalization rates compared with the placebo [12]. This cluster can be effectively managed with measures such as controlling heart rate in patients with AF, optimizing blood pressure control, and implementing lifestyle interventions such as exercise training to enhance functional capacity. When life comes to an end, palliative care, including symptom management and psychological, emotional, and spiritual support, should be properly offered to patients and caregivers throughout the disease course, not only in advanced stages.
2.4. Cluster 4
Cluster 4 was composed of patients who had a median age of 71 years and were identified as having diabetes but not AF. Serum glucose control is the mainstay of this cluster. SGLT2 inhibitors have emerged as a critical component of HFrEF therapy as they possess favorable pleiotropic effects on various body parts such as the kidney, liver, pancreas, blood vessels, and adipose tissue, apart from their primary role as an antidiabetic medication. The EMPEROR-Preserved trial was groundbreaking in the study of HFpEF as it compared the effects of empagliflozin with a placebo in patients with ejection fractions above 40%, irrespective of whether they had diabetes or not. The trial demonstrated a significant reduction in the risk of heart failure-related hospitalizations and cardiovascular mortality, as well as an improvement in renal outcomes.
According to the DELIVER trial, dapagliflozin is superior to placebos in decreasing cardiovascular deaths and hospitalizations due to heart failure in patients with mildly reduced or preserved ejection fractions. Additionally, the study showed that dapagliflozin was effective in patients who previously had ejection fractions below 40% but later saw an increase to over 40%.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are also associated with positive cardiovascular effects. A recent meta-analysis involving 592 patients revealed that liraglutide was connected with significant enhancements in the left ventricular diastolic function.
2.5. Cluster 5
Cluster 5 was a union of Clusters 2 and 4. This cluster had a median age of 82 years, and its members had comorbidities of both diabetes and AF. In the DECLARE-TIMI 58 trial (Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events), dapagliflozin reduced the incidence of AF in patients with diabetes [44]. Efforts for Clusters 2 and 4 should be applied to this cluster, including the ABC pathway for the management of AF and SGLT2i for diabetes.
3. Management of Obesity-Related HFpEF Phenotype
Obesity as a common etiology and co-morbidity for HFpEF has been shown to induce activated sympathetic system and RAAS (and thus hyperaldosteronism with sodium retention) and further promote systemic inflammation [45,46], which may subsequently augment impaired cardiac filling conditions and aggravate unfavorable cardiac remodeling and HF progression [47,48]. Hence, HFrEF patients with central obesity are particularly prone to therapeutic benefits with eplerenone use [49].
Elevated circulating levels of aldosterone, either directly from adipocytes or released from the adrenal gland in response to leptin through the adipokines-cell-signaling molecules secreted (from central obesity or visceral adipose tissue), along with the attenuated anti-aldosterone effects from natriuretic peptides due to an increased neprilysin activity in obesity, may potentiate [50] the deleterious effect of neprilysin HF patients with obesity regardless of HF phenotypes [33]. This “leptin-aldosterone-neprilysin axis activation”, when observed in part as natriuretic peptide deficiency syndrome, as observed in obesity-related HFpEF pathophysiology, may exacerbate the interaction of leptin and aldosterone to promote sodium retention, plasma volume expansion, and regional (such as myocardial) and systemic inflammation and fibrosis (Figure 1) [23,33,43,51].
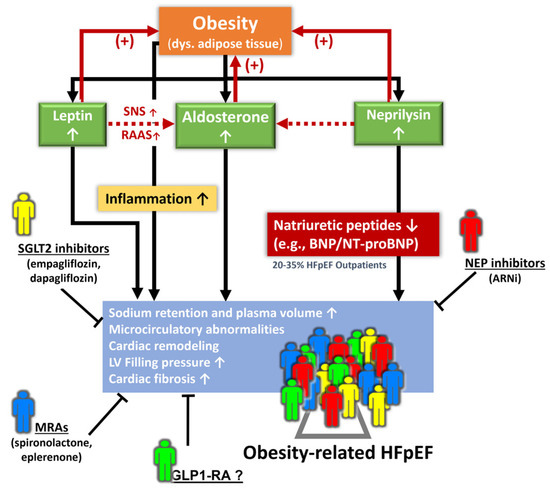
Figure 1. Pathophysiological signaling from the leptin–aldosterone–neprilysin axis activation underlying obesity-related HFpEF and potential diverse phenotypes for pharmacological interventions. ARNi: angiotensin receptor-neprilysin inhibitor; Dys.: dysregulated; GLP1-RA: glucagon-like peptide-1 receptor agonist; MRA: mineralocorticoid receptor antagonist; NEP: neprilysin; RAAS: renin–angiotensin–aldosterone system; SNS: sympathetic nervous system.
Importantly, an activated leptin–aldosterone–neprilysin axis with sustained increases in aldosterone and neprilysin concentration may in turn accelerate the accumulation and inflammation of epicardial fat [52,53]. Recently, proteomics in the LIFE-Heart study (further verified in the Aldo-DHF validation cohort) targeting biomarkers involving volume expansion, myocardial fibrosis, and systemic inflammation has been shown to improve obesity-related HFpEF [54] phenotyping with a distinct biomarker signature. However, whether there may exist some clinical features (e.g., central obesity, region-specific adiposity, e.g., pericardial fat burden) with therapeutic implications using AI-assisted machine learning or clustering may warrant further research (Figure 1).
This entry is adapted from the peer-reviewed paper 10.3390/jpm13050746
This entry is offline, you can click here to edit this entry!
