Patterning, stability, and dispersion of the semiconductor quantum dots (scQDs) are three issues strictly interconnected for successful device manufacturing. Recently, several authors adopted direct optical patterning (DOP) as a step forward in photolithography to position the scQDs in a selected area. However, the chemistry behind the stability, dispersion, and patterning has to be carefully integrated to obtain a functional commercial device.
- semiconductor quantum dots
- ligands
1. Introduction
1.2 The Semiconductor Quantum Dots
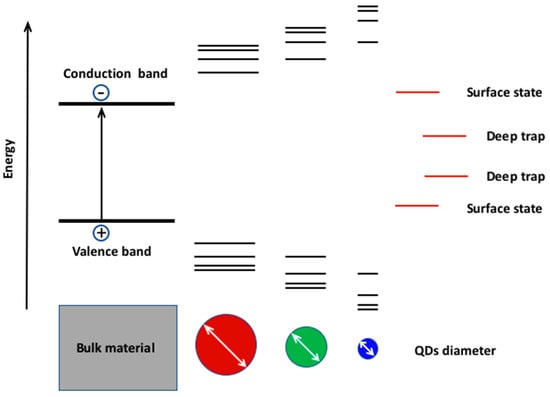
1.3 The Quantum Size Effect and Its Role in the Modulation of the Electro-Optical Properties of the scQDs
1.4 The Core@shell Systems
2 scQD Dispersion: the Ligands and the Surrounding Environment of the scQDs
The tests of dispersion and stability which were carried out, comparing the spreading of the NPLs in three types of polymers (namely: the poly(lauryl methacrylate) (PLMA), the PIB, and the PIB block copolymer (SIBS)) showed the formation of very high transparent films[28], which indicates an optimal dispersion.
Despite a huge amount of work conducted on the study of the organic ligands, it is worth describing also the use of the inorganic ligands. Indeed, they can replace the organic ligands to improve the charge transport between the scQDs[27][28].
Talapin’s group recently showed how the native organic ligand, typically the oleic acid, can be replaced by metal-inorganic salts[27] to obtain intensely luminescent all-inorganic nanocrystals (ILANs). The metal-inorganic salts they used for surface passivation of the scQDs include the metal cations of Cd2+, Zn2+, Pb2+, and In3+ with anions like NO3−, BF4− e triftalate (OTf−).
The role of the metal cations is (i) to remove the native organic ligands, and (ii) to bind the non-metal atom, on the scQD surface. In terms of the Lewis acid-base concept, the ligand metal cation, a Lewis acid, coordinates the electron-rich chalcogenide atom, a Lewis base, at the scQD surface. On the other side, the anion acts as a charge balancer rather than a coordinating agent. The main effect of this ligand exchange is the variation of the scQDs’ solubility (dispersion) of the scQDs in solvents. Indeed, the solubility of the scQDs in non-polar solvents (hexane, toluene, etc.) switches to solubility in polar solvents (DMF, NMF, DMSO, etc.).
3. QD Stability: the Effect of Oxygen and Moisture
Answering the question about the stability of the scQDs under ambient conditions in combination with light will help to adopt the necessary countermeasures to improve the life of any device equipped with this material.
Only recently, the group led by Peng clarified the role of oxygen[29] and water[30] by studying systematically their effect on a well-defined system, the CdSe/CdS core/shell scQDs, in defined experimental conditions in terms of atmosphere (only oxygen, only water, or their defined combination) and different phases at single scQD level or as an ensemble of scQDs in thin film and solution.
The study of the role of oxygen[29] shows that, at the functional level, this molecule maintains the bright state both of the single scQDs and when the scQDs are embedded together in a film (photoactivation). When the oxygen is removed, for example with argon, the scQDs enter a dim state (low emission and small PL shift). The proposed mechanism is that during the photoexcitation, there is the possibility that the scQDs form a trion (two electrons and one hole in the scQDs) bringing the scQDs in the dim charged state (off state). The “bright” state is restored with the presence of oxygen that accept one electron forming the superoxide radical (•O2−). The oxygen reduction returns the scQDs to charge neutrality restoring the scQDs’ optical properties. Another interesting conclusion of this work is that the high quality of the shell avoiding any hole and electron surface traps does not allow any effect of corrosion of the scQDs by the oxygen (the redox potential of the oxygen is quite different from the core/shell scQDs). On the other side, the redox potential of the oxygen should be able to oxidize the surface of bare CdSe scQDs (no core/shell scQDs), especially under photoexcitation producing CdO, SeO2, and CdSeOx [31][32]. The authors conclude that pure oxygen helps to maintain the photophysical properties, and it is not responsible for photo-corrosion of high-quality core/shell nanocrystals. The controversial results found in the literature on the role of oxygen may be due to the non-ideal quality of the prepared core/shell scQDs allowing the corrosion, as reported for the bare CdSe QDs.
Peng’s group studied also the role of water combined with oxygen, showing that this combination is responsible for the corrosion and the loss of the photophysical properties of the scQDs[30]. The complete “story” of the water and oxygen interaction with the scQDs starts with the “ionization by water and deionization by oxygen” step, as reported in Figure 5. In this first step, the excited nanocrystal is negatively ionized (reduced) by water that dissociates, producing a very reactive specie, the hydroxyl radical (•OH) and protons (H+). The negatively charged scQDs are now in the dim state, but the presence of the oxygen, as shown before, brings the scQDs to a neutral state, restoring its bright state and producing as a byproduct the superoxide ion (equilibrium between neutral/bright state and charged/dim state; Figure 5). The presence of the radical species, especially the hydroxyl radical (•OH) formed under the continuous presence of water and irradiation, brings to an acidic pH and a carboxylate ligand detachment from the scQDs’ surfaces. This phenomenon causes the poor solubility of the QDs in the solvent and the precipitation of the bright scQDs (precipitated/bright state, ligand-destructed/bright state; Figure 5). The loss of the surface ligand exposes the inorganic shell to the formation of surface traps bringing further chemical decomposition even of the shell of the scQDs (photo-corrosion)
with irreversible loss of their photophysical properties (etched/bleached state; Figure 5).
It is worth mentioning that when the scQDs are confined in an area with no access to water and oxygen, like in the display applications (the QD’s film is isolated from the environment), the balance between the brightening and dimming states reaches an equilibrium and the decomposition cannot go ahead.
This entry is adapted from the peer-reviewed paper 10.3390/nano13132008
References
- Kim, B.H.; Onses, M.S.; Lim, J.B.; Nam, S.; Oh, N.; Kim, H.; Yu, K.J.; Lee, J.W.; Kim, J.-H.; Kang, S.-K.; et al. High-Resolution Patterns of Quantum Dots Formed by Electrohydrodynamic Jet Printing for Light-Emitting Diodes. Nano Lett. 2015, 15, 969–973.
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor quantum dots: Technological progress and future challenges. Science 2021, 373, eaaz8541.
- Bayer, M. Bridging Two Worlds: Colloidal versus Epitaxial Quantum Dots. Ann. Phys. 2019, 531, 1900039.
- Wu, Y.; Jia, R.; Xu, J.; Song, L.; Liu, Y.; Zhang, Y.; Ullah, S.; Dai, J. Strategies of Improving CsPbX3 Perovskite Quantum Dots Optical Performance. Front. Mater. 2022, 9, 845977.
- Song, Z.; Zhao, J.; Liu, Q. Luminescent perovskites: Recent advances in theory and experiments. Inorg. Chem. Front. 2019, 6, 2969–3011.
- Haydous, F.; Gardner, J.M.; Cappel, U.B. The impact of ligands on the synthesis and application of metal halide perovskite nanocrystals. J. Mater. Chem. A 2021, 9, 23419–23443.
- Efros, A.L.; Brus, L.E. Nanocrystal Quantum Dots: From Discovery to Modern Development. ACS Nano 2021, 15, 6192–6210.
- Weidman, M.C.; Beck, M.E.; Hoffman, R.S.; Prins, F.; Tisdale, W.A. Monodisperse, Air-Stable PbS Nanocrystals via Precursor Stoichiometry Control. ACS Nano 2014, 8, 6363–6371.
- Mocatta, D.; Cohen, G.; Schattner, J.; Millo, O.; Rabani, E.; Banin, U. Heavily Doped Semiconductor Nanocrystal Quantum Dots. Science 2011, 332, 77–81.
- Todescato, F.; Fortunati, I.; Minotto, A.; Signorini, R.; Jasieniak, J.J.; Bozio, R. Engineering of Semiconductor Nanocrystals for Light Emitting Applications. Materials 2016, 9, 672.
- Reiss, P.; Protière, M.; Li, L. Core/Shell Semiconductor Nanocrystals. Small 2009, 5, 154–168.
- Eagle, F.W.; Park, N.; Cash, M.; Cossairt, B.M. Surface Chemistry and Quantum Dot Luminescence: Shell Growth, Atomistic Modification, and Beyond. ACS Energy Lett. 2021, 6, 977–984.
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433.
- Cao, Z.; Shu, Y.; Qin, H.; Su, B.; Peng, X. Quantum Dots with Highly Efficient, Stable, and Multicolor Electrochemiluminescence. ACS Cent. Sci. 2020, 6, 1129–1137.
- Li, J.J.; Wang, Y.A.; Guo, W.; Keay, J.C.; Mishima, T.D.; Johnson, M.B.; Peng, X. Large-Scale Synthesis of Nearly Monodisperse CdSe/CdS Core/Shell Nanocrystals Using Air-Stable Reagents via Successive Ion Layer Adsorption and Reaction. J. Am. Chem. Soc. 2003, 125, 12567–12575.
- Bae, W.K.; Char, K.; Hur, H.; Lee, S. Single-Step Synthesis of Quantum Dots with Chemical Composition Gradients. Chem. Mater. 2008, 20, 531–539.
- Hanifi, D.A.; Bronstein, N.D.; Koscher, B.A.; Nett, Z.; Swabeck, J.K.; Takano, K.; Schwartzberg, A.M.; Maserati, L.; Vandewal, K.; van de Burgt, Y.; et al. Redefining near-unity luminescence in quantum dots with photothermal threshold quantum yield. Science 2019, 363, 1199–1202.
- Meinardi, F.; Colombo, A.; Velizhanin, K.A.; Simonutti, R.; Lorenzon, M.; Beverina, L.; Viswanatha, R.; Klimov, V.I.; Brovelli, S. Large-area luminescent solar concentrators based on ‘Stokes-shift-engineered’ nanocrystals in a mass-polymerized PMMA matrix. Nat. Photonics 2014, 8, 392–399.
- Selopal, G.S.; Abdelkarim, O.; Kumar, P.; Jin, L.; Liu, J.; Zhao, H.; Yurtsever, A.; Vidal, F.; Wang, Z.M.; Rosei, F. Role of Interfacial Engineering of “Giant” Core–Shell Quantum Dots. ACS Appl. Energy Mater. 2022, 5, 1447–1459.
- Carbone, L.; Nobile, C.; De Giorgi, M.; Sala, F.D.; Morello, G.; Pompa, P.; Hytch, M.; Snoeck, E.; Fiore, A.; Franchini, I.R.; et al. Synthesis and Micrometer-Scale Assembly of Colloidal CdSe/CdS Nanorods Prepared by a Seeded Growth Approach. Nano Lett. 2007, 7, 2942–2950.
- Srivastava, A.K.; Zhang, W.; Schneider, J.; Halpert, J.E.; Rogach, A.L. Luminescent Down-Conversion Semiconductor Quantum Dots and Aligned Quantum Rods for Liquid Crystal Displays. Adv. Sci. 2019, 6, 1901345.
- Diroll, B.T.; Guzelturk, B.; Po, H.; Dabard, C.; Fu, N.; Makke, L.; Lhuillier, E.; Ithurria, S. 2D II–VI Semiconductor Nanoplatelets: From Material Synthesis to Optoelectronic Integration. Chem. Rev. 2023, 123, 3543–3624.
- Onal, A.; Sadeghi, S.; Melikov, R.; Karatum, O.; Eren, G.O.; Nizamoglu, S. Quantum Dot to Nanorod Transition for Efficient White-Light-Emitting Diodes with Suppressed Absorption Losses. ACS Photonics 2022, 9, 3268–3278.
- Ehlert, S.; Stegelmeier, C.; Pirner, D.; Förster, S.; A General Route to Optically Transparent Highly Filled Polymer Nanocomposites. Macromolecules 2015, 48, 5323-5327, .
- Shiman, D.I.; Sayevich, V.; Meerbach, C.; Nikishau, P.A.; Vasilenko, I.V.; Gaponik, N.; Kostjuk, S.V.; Lesnyak, V.; Robust Polymer Matrix Based on Isobutylene (Co)polymers for Efficient Encapsulation of Colloidal Semiconductor Nanocrystals. ACS Appl. Nano Mater. 2019, 2, 956-963, .
- Reitinger, N.; Hohenau, A.; Köstler, S.; Krenn, J.R.; Leitner, A.; Radiationless energy transfer in CdSe/ZnS quantum dot aggregates embedded in PMMA. Phys. Status Solidi A 2011, 208, 710-714, .
- Xiao, P.; Zhang, Z.; Ge, J.; Deng, Y.; Chen, X.; Zhang, J.-R.; Deng, Z.; Kambe, Y.; Talapin, D.V.; Wang, Y.; et al. Surface passivation of intensely luminescent all-inorganic nanocrystals and their direct optical patterning. Nat. Commun. 2023, 14, 49, .
- Kovalenko, M.V.; Scheele, M.; Talapin, D.V.; Colloidal Nanocrystals with Molecular Metal Chalcogenide Surface Ligands. Science 2009, 324, 1417-1420, .
- Hu, Z.; Liu, S.; Qin, H.; Zhou, J.; Peng, X.; Oxygen Stabilizes Photoluminescence of CdSe/CdS Core/Shell Quantum Dots via Deionization. J. Am. Chem. Soc. 2020, 142, 4254-4264, .
- Hu, Z.; Shu, Y.; Qin, H.; Hu, X.; Peng, X.; Water Effects on Colloidal Semiconductor Nanocrystals: Correlation of Photophysics and Photochemistry. J. Am. Chem. Soc. 2021, 143, 18721-18732, .
- Carrillo-Carrión, C.; Cárdenas, S.; Simonet, B.M.; Valcárcel, M.; Quantum dots luminescence enhancement due to illumination with UV/Vis light. Chem. Commun. 2009, 35, 5214-5226, .
- Hines, D.A.; Becker, M.A.; Kamat, P.V.; Photoinduced Surface Oxidation and Its Effect on the Exciton Dynamics of CdSe Quantum Dots. J. Phys. Chem. C 2012, 116, 13452-13457, .
