Micro and nanoparticles are not only understood as components of materials but as small functional units too. Particles can be designed for the primary transduction of physical and chemical signals and, therefore, become a valuable component in sensing systems. Due to their small size, they are particularly interesting for sensing in microfluidic systems, in microarray arrangements and in miniaturized biotechnological systems and microreactors, in general. Here, an overview of the recent development in the preparation of micro and nanoparticles for sensing purposes in microfluidics and application of particles in various microfluidic devices is presented. The concept of sensor particles is particularly useful for combining a direct contact between cells, biomolecules and media with a contactless optical readout. In addition to the construction and synthesis of micro and nanoparticles with transducer functions, examples of chemical and biological applications are reported.
- sensors
- nanoparticles
- microfluidic
1. Introduction
The development of micro and nanoparticles is not only motivated by the desire for new materials, but mainly driven by particle-specific functional properties. Functional micro and nanoparticles are more than parts of a material but can be applied as miniaturized individual system components. These tiny objects can connect classical features of a new material with the ability to act like a miniaturized device. Thus, the receiving of signals, signal transduction and emission are tasks which can be carried out by particles with transducer properties [1]. Particularly interesting is the search for particles which are able to serve as localized sensors for conversion of chemical or biomolecular information into a physically readable signal for contactless transmission.
A special need for small-sized sensors arises from the development of microreaction technology and microfluidic systems for chemical and biological applications [2]. These devices work with the smallest volumes of liquids typically in the sub-microliter as well as the nanoliter range [3]. For local measurements in such fluidic systems, conventional chemical sensors and analytical devices are much too large and, therefore, not applicable. The analytical or sensing tasks in such systems demand for transducers in the dimensions between parts of a millimeter down to the micrometer or nanometer level.
At first sight, the miniaturization seems to be connected with difficulties which are associated with the shrinking of geometric dimensions. However, the introduction of particle-based transduction opens new possibilities, too. These are related to the special functional properties of the applied particles and the fact that specific physical effects can become usable for the conversion of signals in the lower micron and, in particular, in the nanometer range. This concerns, for example, size- and shape-dependences of optical properties of particles offering advantages for surface-enhanced Raman scattering (SERS) or so-called plasmonic sensing [4]. In addition to microchambers, microchannels and microreactors, the implementation of miniaturized sensors in microdroplets is particular challenging. During recent years, it could be shown that micro and nanosensor particles are well suited for the read-out of analytical information from tiny droplets [5] and microfluid segments [6].
2. Application of Sensor Particles for Temperature, pH and Oxygen Measurements in Microfluidic Systems
2.1. Temperature Sensing
Temperature control is among the important functionalities, which could be integrated in a microfluidic device. By applying non-invasive spectroscopic techniques, temperature can be monitored at multiple locations inside microfluidic devices [7].
In order to efficiently perform various chemical as well as biochemical reactions and assays, it is very important to control temperature properly and accurately. Polymerase chain reaction (PCR) is among the examples of a temperature-controlled process, which requires an accurate temperature measurement, and is widely used for DNA sequencing, pathogen detection and genetic analysis. D. C. Leslie et al. [8] presented an interferometric temperature control system with closed-loop temperature heating and cooling for direct temperature measurement in order to monitor PCR process. Non-contact temperature sensing was performed using Extrinsic Fabry–Perot interferometry (EFPI). A schematic representation of an interferometric temperature control system is shown in Figure 9. During the experiment, optical fiber emitted the NIR radiation (850 nm) into the to the microfluidic chamber, whose surfaces were modified with a platinum nanoparticle monolayer. Interference pattern was formed by reflected lights, which were collected from the upper and lower chamber surfaces, and was used for determining the optical path length. By monitoring the change in the optical path length, the temperature inside the chamber was determined because the refractive index of the medium decreased with increasing temperature. The presented interferometric temperature control system was applied in the PCR process for amplification of a portion λ-phage DNA and human genomic DNA. As a result, the temperature was monitored with detectable temperature change of 0.3 °C. This universal thermocyling system can be applied for a variety of disposable miniaturized platforms such as cell-based devices or sequencing tools.
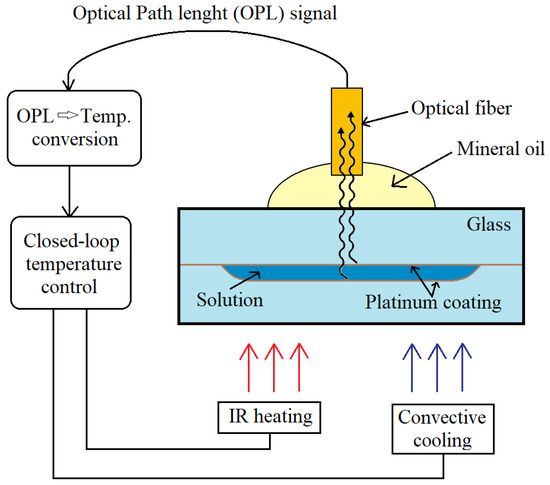
Figure 9. Schematic representation of interferometric temperature control system. Adapted from [8]. Copyright 2012 © The Royal Society of Chemistry.
Separate fluorescent dyes in solution can also be used as temperature indicators. Generally, not only quenching agents but also changes in pH can strongly affect spectroscopic properties of the dyes; therefore, it is challenging to measure temperature precisely. Another disadvantage of fluorescent dye application is that their fluorescence properties depend on the temperature linearly only in a narrow temperature range [7]. Moreover, the concentration of fluorescent dyes influences the final fluorescence intensity. Therefore, the ratiometric technique, which is based on the measurement of intensities at two or more peaks of an excitation or emission spectrum and does not depend of temperature probe concentration, can be applied for temperature monitoring.
2.2. pH Sensing
In addition to the temperature control, the information about ion concentrations—for example, pH—is very important due to the fact that medium pH can strongly affect many metabolic processes and enzymatic reactions. Additionally, the intracellular pH is involved in cellular events such as cell growth, endocytosis, transport of ions, activity of enzymes and evolving of some diseases—for example, cancer [9]. O. Kreft et al. [10] presented a pH sensor system based on polymer capsules for monitoring pH changes in human breast cancer cells and fibroblast environments. Multifunctional capsules were formed by incorporating magnetite nanoparticles and pH-indicator dye into a polymer microcapsule shell. SNARF-1-dextran fluorophore was incorporated in spherical CaCO3 particles by a coprecipitation method [9]. Later, SNARF-1-loaded particles were embedded in the shells of polyelectrolytes. A multi-layered (onion-like) shell was formed by consecutively depositing 10 layers of polystyrene sulfonate (PSS) and polyallylamine hydrochloride (PAH). Therefore, SNARF-1-dextran fluorophore was retained in the microcapsule shell and was not dispersed throughout the sample. A pH-sensitive fluorophore exhibited a significant emission shift under different pH conditions: in acidic medium, dye showed a green emission and in basic medium, the fluorescence peak was at the red spectral region. Due to the incorporation of magnetite nanoparticles, it was possible to transport microcapsules to a specific location or easily separate them from the sample by applying magnetic field. In order to observe local pH changes under cell culture conditions, cancer cells and fibroblasts were incubated with sensor capsules. During endocytic uptake, capsules got inside the cells, and fluorescence measurements proved that inside the endosomal/lysosomal compartments medium was acidic and did not depend on the varied pH value outside the cells. Microcapsules, which were left outside the cells, corresponded well to the pH changes. However, the distribution of SNARF-dextran in microcapsules was inhomogeneous and the amount of dye varied in different capsules. Moreover, due to photobleaching, the absolute fluorescence intensity decreased.
Another microfluidic platform for pH monitoring was presented by M. P. Gashti et al. [11]. By applying a fluorescent nanoparticle-based sensor, ratiometric pH imaging of oral bacteria Streptococcus salivarius biofilms was performed in real-time. For this purpose, Ag@SiO2 + FiTC nanoparticles were covalently patterned on a planar glass slide and formed a thin sensing surface that featured good photostability and ability to preserve Ag@SiO2 + FiTC nanoparticles under flow conditions. Streptococcus salivarius bacteria were used for co-aggregation, surface attachment and formation of biofilm directly at the sensing surface inside a Y-channel microfluidic device due to hair-like protein-based appendages. In this study, fluorescein was used as a pH-sensitive fluorescent dye and was covalently attached inside the 15 nm silica layer for quantitative pH measurements. It was demonstrated that the glucose concentration changes in the nutrient solution stimulated pH changes at the attachment surface of a biofilm, which were monitored by ratiometric pH images. Due to good biocompatibility, it was possible to perform experiments, which lasted longer than one week. Additionally, density and porosity of biofilms were investigated by varying flow rates of the nutrient solution and monitoring the pH changes.
In order to monitor pH changes or determine the pH value more precisely, luminescence lifetime-based measurements offer this opportunity since the lifetime of a fluorophore does not depend on the fluorescence intensity or wave-length interferences. The integrated pH sensor microstructures for time-domain dual lifetime referencing (t-DLR) measurements in microfluidic channels were presented by E. Poehler et al. [12] and applied in miniaturized electrophoretic procedures (μFFIEF). For preparation of a luminescent sensor, pH indicator 5(6)-carboxyfluorescein N-hydroxy-succinimide ester (CF) was covalently bonded to the pHEMA copolymer and dissolved in ethanol. The mixture was added to aqueous solution that contained Ru(dpp)3 embedded PAN reference nanoparticles and the polymer ink was formed. The polymer ink was inkjet-printed on a glass slide rapidly with a low sample consumption. Then, the glass slide was integrated into a microfluidic chip. The sensor was used in order to separate and determine three different proteins (β-lactoglobulin A, conalbumin and myoglobin), which were labelled with red fluorescent dye P503 (Py1). The pH sensor worked in the pH range from 4 to 8 and showed fast response times. The microfluidic chip demonstrated very high stability during measurements under flow conditions with flow rate of 50 μL/min. In the t-DLR scheme, a signal, which was proportional to the pH value, was estimated from the ratio of the two images, which were taken in different time moments. The first image was made with 5 μs exposure time under LED irradiation. It provided information about the luminescence decay times of pH indicator and reference dye. After illumination was stopped and delay of 1 μs, another image was taken and collected the information only about the emission decay of reference dye. Due to the fact that lifetime-based ratiometric pH sensing was independent of background noises, it provided more accurate data. However, the pH sensor calibration was valid for a limited time due to the decreased intensity caused by applied constant LED illumination for 30 min.
2.3. Oxygen Sensing
The concept of oxygen sensing and imaging is preferably based on the quenching of oxygen-sensitive dye luminescence by molecular oxygen. Due to quenching, the intensity of luminescence decreases, and changes in decay time occur. Intensity-based phosphorescence or fluorescence measurements are widely used for their simplicity, low cost and easily accessible equipment. However, these measurements are sensitive to excitation intensity and efficiency of detection system. Moreover, many other factors such as photobleaching, uneven dye distributions, scattering of excitation or emission light can strongly affect luminescence intensity. Therefore, it is necessary to apply referenced detection schemes—for example, the ratiometric method. During ratiometric fluorescence measurement, the intensities at two or more emission peaks of an oxygen-sensitive indicator and a reference dye are measured in order to detect changes in oxygen concentration. By applying this method, environment variations are reduced. Nonetheless, signal varieties due to photobleaching, leaching of fluorescent dye or Rayleigh scattering still have an impact on precise analyte measurement [13].
Another widely applied technique is fluorescence lifetime imaging, which is based on determination of the spatial distribution of fluorescence lifetimes at every pixel of the image. As the fluorescence lifetime of a fluorophore does not depend on its concentration, in the first approximation, the thickness of the sample, excitation intensity or photobleaching do not disturb the measurement seriously. The lifetime-based measurements provide information about the molecular environment of the fluorophore such as pH, concentration of oxygen and molecular binding. Fluorescence lifetime imaging techniques can be classified into frequency-domain and time-domain techniques. In the time-domain techniques, analyte is excited by short light and the fluorescence emission is recorded as a function of time. In the frequency domain, modulated source of light excites the sample and the fluorescence emission becomes modulated and phase-shifted from the excitation curve; therefore, fluorescence lifetimes are calculated from the observed phase-shift and modulation [14].
By combining the oxygen imaging with microfluidics systems, it is possible to visualize concentration gradients of analyte, evaluate concentrations, monitor chemical reactions and perform single cell analysis [13]. In order to investigate metabolism and growth of aerobic bacteria in droplet bioreactor systems, it is critical to monitor the oxygen concentration inside the droplets.
Measurements of absolute oxygen concentration and optical density (OD) during incubation of Escherichia coli and M. smegmatis bacteria inside the droplets were performed and presented in the research of M. Horka et al. [15]. In order to form an oxygen sensor, phosphorescent indicator dye (PtTPTBPF) was immobilized in core−shell structured poly-(styrene-block vinylpyrrolidone) (PSPVP) nanobeads. Due to the core−shell structure, sensor particles were dispersed in aqueous phase and oxygen-sensitive dyes were immobilizes in the lipophilic core of the nanobead. For phosphorescence frequency-domain lifetime measurements, the nanoparticles were excited by red light and due to large Stokes shift, particles emitted near-infrared (NIR) light. Fluorescence emission in the NIR region offered the ability to use PSPVP-PtTPTBPF particles in biological samples because of minimized background fluorescence. The most significant effect was observed when a large quantity of oxygen was consumed during the exponential growth phase of bacterial colony, which resulted in the significantly decreased concentration of oxygen in the droplets. The absolute oxygen concentration was accurately determined with a resolution of 0.07–0.12 μM.
Non-invasive, optical monitoring of oxygen concentration during bacterial cultivation in microfluidic droplet-based system was presented by J. Cao et al. [6]. Dissolved oxygen concentration, cell density and endogenous autofluorescence intensity were simultaneously sensed in nl-sized droplets by utilizing a microflow-through fluorimeter and photometer. For that purpose, polystyrene beads (PS) doped with oxygen-sensitive dye PtTPTBP were mixed with culture liquid in microfluid segments. Two bacterial strains Streptomyces acidiscabies E13 and Psychrobacillus psychrodurans UrPLO1, which were resistant to heavy metal ions, were investigated. A model of the microfluidic experimental setup is shown schematically in Figure 10A. Microfluid droplets were generated through PEEK 7-port manifold. A syringe pump system controlled 5 syringes filled with the effector CuCl2, suspension of sensor beads, cultivation medium, cell suspension and carrier liquid PP9. By varying flow rates of the effector solution and the cultivation medium, reactants were dosed and, therefore, it was possible to tune the composition of the microfluid segments inside a large sequence. After generation of micro fluid segments, they were transported with a fixed 55 μL/min flow rate to the detection unit (Figure 10B). For determining a distance between two segments, droplet size and number, light scattering measurements were performed by using two diodes with peak wavelengths of 470 and 505 nm. For evaluation of the bacterial growth and the oxygen consumption, the sample was excited under visible light with wavelengths of 470 and 405 nm. The results showed that the phosphorescence intensity measurements were sufficient for monitoring oxygen concentration inside the droplets. During microtoxicological investigation on the effect of copper (II) chloride, it was proved that the investigated strain of Psychrobacillus psychrodurans UrPLO1 had a strong tolerance to Cu2+. However, uneven distribution of the dye-labelled sensor particles inside the droplet, photobleaching and also droplet geometry could have a relatively high impact on the accuracy of an intensity-based luminescence measurements.
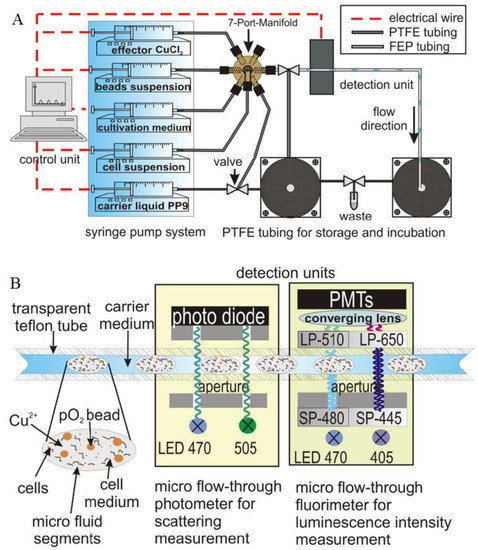
Figure 10. (A) Model of the microfluidic experimental set-up. (B) Schematic illustration of the optical detector units that consisted of channel microflow-through photometry and fluorimetry. Reprinted from [6] with permission from Springer-Verlag Wien 2014.
Y. Yabuki et al. [16] proposed a non-toxic and contactless technique for estimating the absolute values of oxygen concentration in a cell culture microfluidic device. Pd-octaethylporphine (Pd-OEP) dye loaded polystyrene microparticles were embedded in dimethylpolysiloxane (PDMS) by creating a thin oxygen sensing film in order to avoid direct contact of the dyes with the living cells. After the oxygen-sensing film was installed into the microfluidic device, the 4T1 cells were cultured on it. Due to this, monitoring of oxygen consumption was performed stably and over long periods of time. It was determined that the response time of the oxygen concentration changes depended on microparticle size and oxygen-sensing film thickness. The smaller microparticles and thicker film led to the shorter response time. Due to the fact that phosphorescence lifetime depends on the oxygen concentration, pO2 changes were quantified from the lifetime/phosphorescence quantum yield, respectively, and the oxygen consumption quantity was estimated from pO2 shifts. It was shown that the oxygen gradient downshifted due to cellular respiration process. In addition, by transplanting tumor fragments, which were excised from the mouse, on the sensor film, oxygen consumption was screened at various oxygen concentrations. As a result, oxygen concentration decreased in the parts in which the tumor tissue fragments were placed.
L. C. Lasave et al. [17] presented a simple and low-priced method for integrating an oxygen-sensitive sensor particle into a glass microfluidic device. A sensing strategy relied on the formation of a sensing layer by physical adsorption of nanoparticles. The formed sensing layer produced good stability, very fast response time (less than 0.2 s) and optimum sensitivity. Conjugated polymeric nanoparticles had a hydrophobic polyfluorene poly(fluorene-co-benzothiadiazole) backbone that contained covalently attached Pt (II) (benzo)porphyrins. In this research, microfluidic chips channels were coated with sensor nanoparticles, which luminescence was modulated by oxygen concentration. For increasing roughness and enhancing adsorbent, the surface area of smooth microchannel, made from glass, was increased by in situ generation. Finally, the developed techniques were investigated for monitoring red-ox (oxidation of glucose) reaction using a packed-bed reactor (Figure 11). For this purpose, silica particles, which contained the immobilized glucose oxidase (GOx) enzyme, were added into the microfluidic chip with integrated oxygen sensors. By applying a strong water flow in the outlets, GOx -carrying silica particles were removed from the chip; therefore, the microfluidic device could be reused several times.
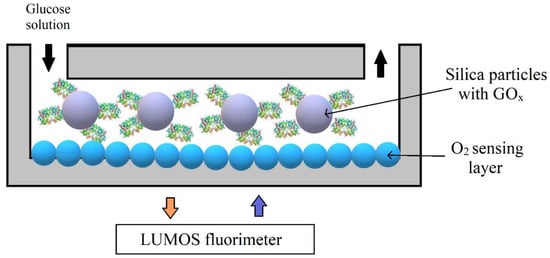
Figure 11. Schematic representation of the experimental setup for monitoring an enzymatic activity in microfluidic reactors with oxygen sensing layer. Adapted from [18]. Copyright © 2015 The Royal Society of Chemistry.
2.4. Particle-Base Imaging of Oxygen Distribution
Oxygen imaging using a fluorescence microscope was investigated by B. Ungerböck et al. [19] Oxygen measurements were carried out inside a Y-shaped microfluidic reactor by applying luminescent indicator dye doped nanoparticles. Sensor particles were prepared by staining poly(styrene-block-vinylpyrrolidone) (PSPVP) beads with oxygen-sensitive luminescent dye Ir(Cs)2(acac) according to the report of S. M. Borisov and I. Klimant [20]. Dissolved oxygen concentrations were measured during the glucose oxidation reaction in the presence of glucose oxidase. By comparing the obtained experimental results and predictions of model simulations, good agreement was found, which showed a high measurement accuracy. The accuracy of the presented oxygen-imaging technique was 0.03, and it was evaluated by comparing a calculated standard deviation to a curve fitted to experimental data.
Later on, B. Ungerböck et al. [21] presented magnetic optical sensor particles (MOSePs) for monitoring the enzymatic activity of glucose oxidase and cellular respiration. MOSePs were produced by an optimized nanoprecipitation method. In order to prepare sensor particles, PSMA nanoparticles were doped with magnetite nanoparticles and different luminescent dyes (PtTFPP, MFY or Ir(Cs)2(acac)). By using a magnet, the particles were collected and accumulated in one place inside a microfluidic channel in order to form sensor spots. In situ formed sensor spots featured good stability at flow rates typically applied in microfluidic systems. Among biggest advantages was an ability to easily change the location of sensor spot by moving the magnet outside a microfluidic channel. By forming sensor spots, enzymatic activity of glucose oxidase was monitored by detecting pO2 along the channel, during the glucose oxidation. Respiratory cell activity was investigated by using a suspension of MOSePs containing Ir(Cs)2(acac), when E. coli were incubated inside a microfluidic chip. As a result, the pO2 image showed the oxygen concentration was the lowest at places in the chip where cell aggregates were located.
2.5. Combination of Particle-Based Oxygen Sensing with Flow Velocimetry
H. D. Kim et al. [22] used a conventional microparticle image velocimetry method for performing simultaneous measurements of dissolved oxygen concentration (DOC) and velocity fields. DOC was determined by applying oxygen-sensitive particles, which were produced by the dispersion polymerization method. Polystyrene (PS) particles were doped with PtOEP dye, whose phosphorescence was quenched by oxygen molecules. Therefore, formed oxygen-sensitive particles were applied not only as tracer particles but also as oxygen sensors. In order to observe a diffusion of dissolved oxygen in a Y-shaped microchannel, oxygen-sensitive particle were dispersed in de-ionized water with DOC values of 0 or 100%. Particles were excited at 385 nm, and their phosphorescence intensity distribution was recorded. As a result, due to different dissolved oxygen concentrations in water, it was possible to observe a qualitative visualization of two different intensities of phosphorescence. It was found that the maximum velocity of the water streams was 3.5 mm/s, and a parabolic velocity profile was obtained. Additionally, it was determined that after 3 s exposure to excitation light, photobleaching of particles was less than 2%.
2.6. Combination of Oxygen and pH Sensing
Simultaneous determination of oxygen concentration and pH during the enzymatic reaction was presented by J. Ehgartner et al. [23] Contactless and inexpensive detection was enabled by using poly(styrene-block-vinylpyrrolidone) (PSPVP) core−shell nanoparticles, whose average diameter was 180 nm. Due to the core−shell structure, the oxygen-sensitive dye PtTPTBPF was incorporated into the particle’s core and a pH-sensitive dye (aza-BODIPY) was captured into the polyvinylpyrrolidone shell. Modified dual lifetime referencing (m-DLR) was used to sense pH and oxygen in microfluidic system. After the sinusoidally modulated light source excited the indicators, phase shift as well as luminescence emission were recorded. The detection method was based on the overall phase shift measurement of pH and oxygen sensitive luminescence dyes at two different modulation frequencies. It was necessary that luminescent lifetimes of indicators were in different time ranges. In this research, an oxygen-sensitive dye had a relatively long phosphorescence lifetime in the microsecond range when a pH indicator had a shorter fluorescence lifetime in the nanosecond range. The presented system offered the advantage that it used a red-light excitation at 620 nm, which caused decreased background fluorescence and scattering. Additionally, it possessed higher photostability and opens the possibility to use sensor nanoparticles in microfluidic devices without additional integration. In order to monitor pH changes in real-time, Penicillin G acylase catalyzed an enzymatic conversion of Penicillin G into 6-aminopenicillanic acid and phenyl acetic acid was investigated. By comparing results between the simulation and experiment, it was shown that experimental data were in great agreement with the expected simulation results. It was found that oxygen concentrations were determined at a resolution from approximately 0.02 to 0.32 mg/L. The resolution of pH value varied from 0.03 to 0.1 pH units.
3. Conclusions
Nanoparticles are widely investigated and attract a lot of attention due to their optical, thermal, magnetic and electrical properties and their potential to be utilized for a wide variety of applications including temperature control, pH sensing, oxygen imaging, detection of organic molecules or inorganic ions and biosensing. By using microfluidics devices, micro and nanoparticles and their composites can be synthesized in a controllable and reproducible manner. Moreover, by applying microfluidic technology, it is possible to handle microscale or nanoscale fluids for sensing purposes and reduce the amount of consumed reagent. Among the different methods, which are used for sensing, optical (fluorescence, surface plasmon resonance-based optical detection) and electrochemical methods are the most frequently applied for their selectivity and sensitivity.
This entry is adapted from the peer-reviewed paper 10.3390/app10238353
References
- Kohler, M.A. Mobile Microspies: Particles for Sensing and Communication, 1st ed.; Jenny Stanford Publishing: Beijing, China, 2018.
- Ma, J.; Lee, S.M.-Y.; Yi, C.; Li, C.-W. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications—A review. Lab Chip 2017, 17, 209–226.
- Kumacheva, E.; Garstecki, P. Microfluidic Reactors for Polymer Particles; Wiley-Blackwell: Oxford, UK, 2011.
- Kim, J. Joining plasmonics with microfluidics: From convenience to inevitability. Lab Chip 2012, 12, 3611.
- Guo, Z.-X.; Zeng, Q.; Zhang, M.; Hong, L.-Y.; Zhao, Y.-F.; Liu, W.; Guo, S.-S.; Zhao, X.-Z. Valve-based microfluidic droplet micromixer and mercury (II) ion detection. Sens. Actuators A Phys. 2011, 172, 546–551.
- Cao, J.; Nagl, S.; Kothe, E.; Köhler, J.M. Oxygen sensor nanoparticles for monitoring bacterial growth and characterization of dose–response functions in microfluidic screenings. Microchim. Acta 2015, 182, 385–394.
- Ebert, S.; Travis, K.; Lincoln, B.; Guck, J. Fluorescence ratio thermometry in a microfluidic dual-beam laser trap. Opt. Express 2007, 15, 15493.
- Leslie, D.C.; Seker, E.; Bazydlo, L.A.L.; Strachan, B.C.; Landers, J.P. Platinum nanoparticle-facilitated reflective surfaces for non-contact temperature control in microfluidic devices for PCR amplification. Lab Chip 2012, 12, 127–132.
- Petrov, A.I.; Volodkin, D.V.; Sukhorukov, G.B. Protein-Calcium Carbonate Coprecipitation: A Tool for Protein Encapsulation. Biotechnol. Prog. 2008, 21, 918–925.
- Kreft, O.; Javier, A.M.; Sukhorukov, G.B.; Parak, W.J. Polymer microcapsules as mobile local pH-sensors. J. Mater. Chem. 2007, 17, 4471.
- Gashti, M.P.; Asselin, J.; Barbeau, J.; Boudreau, D.; Greener, J. A microfluidic platform with pH imaging for chemical and hydrodynamic stimulation of intact oral biofilms. Lab Chip 2016, 16, 1412–1419.
- Poehler, E.; Herzog, C.; Suendermann, M.; Pfeiffer, S.A.; Nagl, S. Development of microscopic time-domain dual lifetime referencing luminescence detection for pH monitoring in microfluidic free-flow isoelectric focusing. Eng. Life Sci. 2015, 15, 276–285.
- Sun, S.; Ungerböck, B.; Mayr, T. Imaging of oxygen in microreactors and microfluidic systems. Methods Appl. Fluoresc 2015, 3, 034002.
- Becker, W. Fluorescence lifetime imaging—techniques and applications. J. Microsc. 2012, 247, 119–136.
- Horka, M.; Sun, S.; Ruszczak, A.; Garstecki, P.; Mayr, T. Lifetime of Phosphorescence from Nanoparticles Yields Accurate Measurement of Concentration of Oxygen in Microdroplets, Allowing One To Monitor the Metabolism of Bacteria. Anal. Chem. 2016, 88, 12006–12012.
- Yabuki, Y.; Iwamoto, Y.; Tsukada, K. Micro/nano particle-based oxygen sensing film for monitoring respiration of cells cultured in a microfluidic device. Jpn. J. Appl. Phys. 2019, 58, SDDK03.
- Lasave, L.C.; Borisov, S.M.; Ehgartner, J.; Mayr, T. Quick and simple integration of optical oxygen sensors into glass-based microfluidic devices. RSC Adv. 2015, 5, 70808–70816.
- Wieduwilt, T.; Zeisberger, M.; Thiele, M.; Doherty, B.; Chemnitz, M.; Csaki, A.; Fritzsche, W.; Schmidt, M.A. Gold-reinforced silver nanoprisms on optical fiber tapers—A new base for high precision sensing. APL Photonics 2016, 1, 066102.
- Ungerböck, B.; Pohar, A.; Mayr, T.; Plazl, I. Online oxygen measurements inside a microreactor with modeling of transport phenomena. Microfluid. Nanofluidics 2013, 14, 565–574.
- Borisov, S.M.; Klimant, I. Luminescent nanobeads for optical sensing and imaging of dissolved oxygen. Microchim. Acta 2009, 164, 7–15.
- Ungerböck, B.; Fellinger, S.; Sulzer, P.; Abel, T.; Mayr, T. Magnetic optical sensor particles: A flexible analytical tool for microfluidic devices. Analyst 2014, 139, 2551–2559.
- Kim, H.D.; Yi, S.J.; Kim, K.C. Simultaneous measurement of dissolved oxygen concentration and velocity field in microfluidics using oxygen-sensitive particles. Microfluid. Nanofluidics 2013, 15, 139–149.
- Ehgartner, J.; Strobl, M.; Bolivar, J.M.; Rabl, D.; Rothbauer, M.; Ertl, P.; Borisov, S.M.; Mayr, T. Simultaneous Determination of Oxygen and pH Inside Microfluidic Devices Using Core–Shell Nanosensors. Anal. Chem. 2016, 88, 9796–9804.
