Classical microextraction techniques such as SPME, SBSE, and TFME preferentially employ highly viscous pristine polymeric sorbents including PDMS, PEG, and PA, etc., as the extracting phase. During extraction, the analytes are solvated by the extracting polymeric phase. The diffusion coefficient in the highly viscous polymeric coating enables the analytes to penetrate the whole volume of the coating if enough time is allowed. As such, the mass transfer rate as well as the extraction kinetic is relatively slow in the viscous polymeric sorbent coating. When the analytes are heavier (high molar mass), the diffusion into the polymeric extracting phase is even slower. The extraction kinetic can be enhanced by impregnating the viscous polymeric phases with high surface area carbonaceous particulates material such as divinyl benzene (DVB) and Carboxen. These particles act as a bridge inside the liquid polymeric phases and facilitate faster extraction kinetics.
Fabric phase sorptive extraction is the only microextraction technique that offers a complete range of sorbent chemistries including polar, medium polar, nonpolar, cation exchanger, anion exchanger, mixed mode, zwitterionic, as well as zwitterionic mixed mode sorbents. Table 1 provides a list of major FPSE sorbent chemistries. It should be noted that all of these sorbents can be coated either on 100% cotton cellulose (hydrophilic) or on fiber glass (neutral) or on polyester (hydrophobic) substrates.
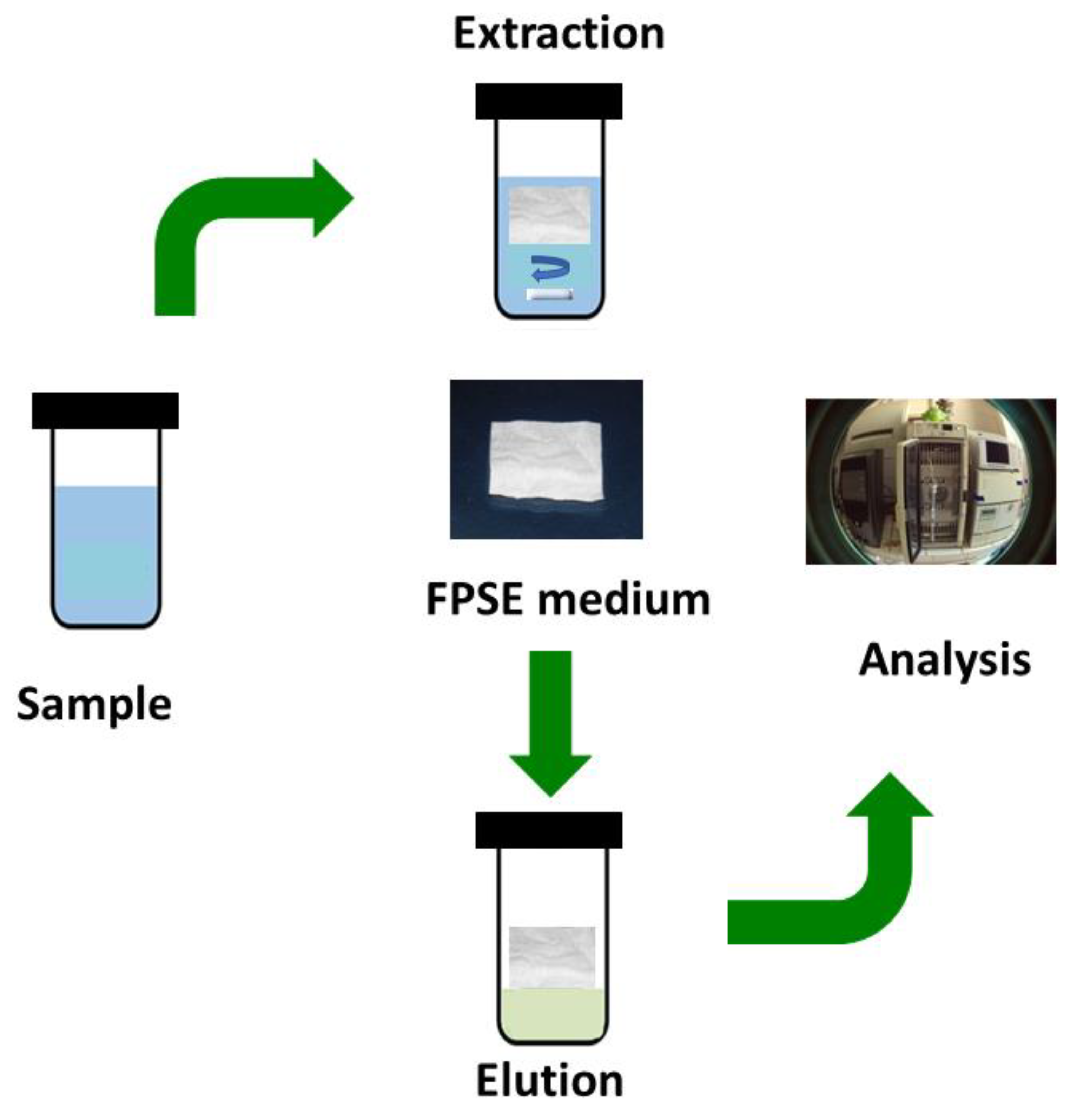
Unlike solid phase microextraction and similar sorbent based microextraction techniques, method development in fabric phase sorptive extraction is simple and straight forward. Figure 1 presents a graphical schematic of a typical FPSE workflow.
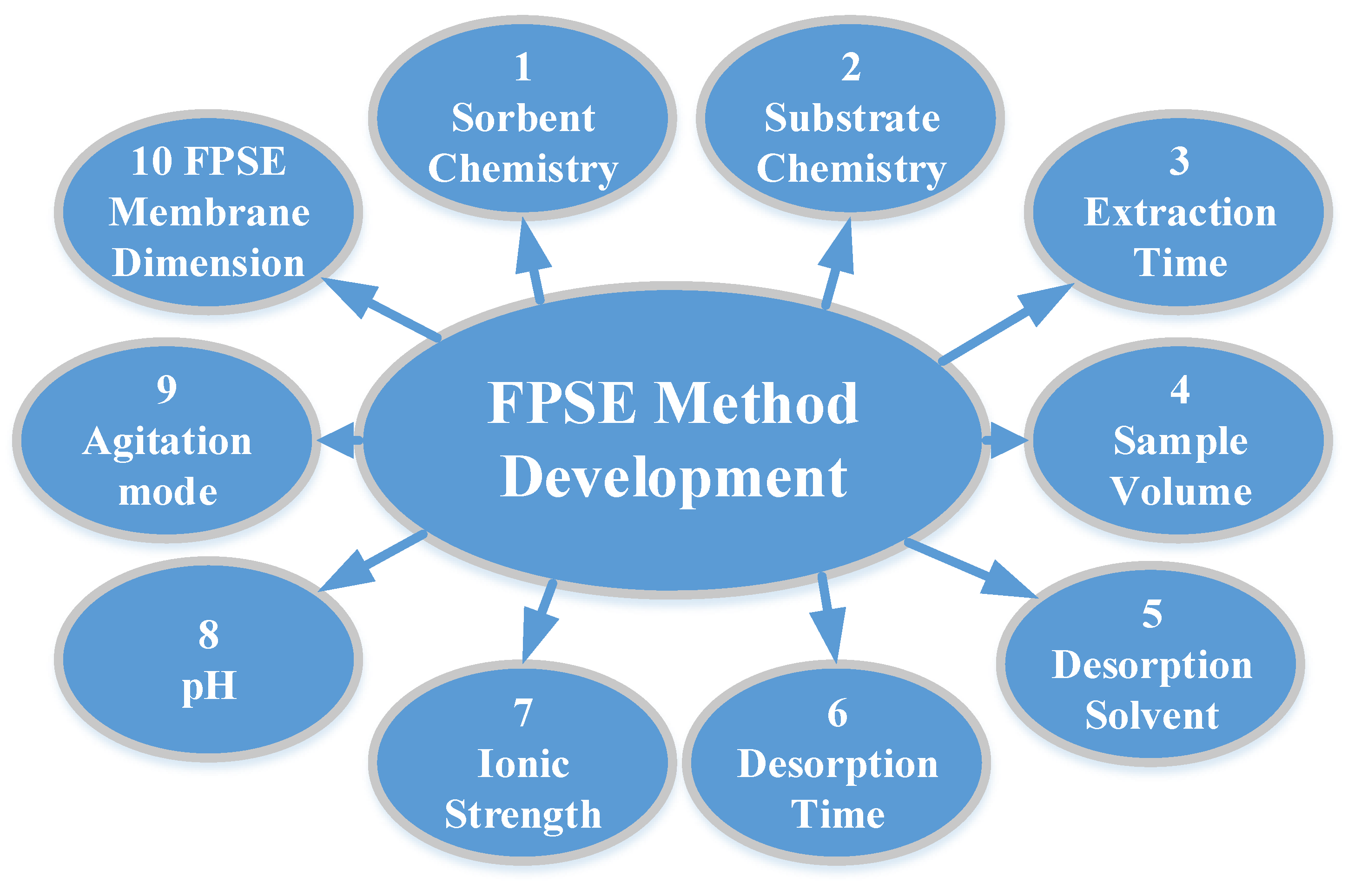
FPSE does not require any sample pre-treatment process to reduce/minimize matrix interferents such as filtration, protein precipitation, or centrifugation, and the FPSE membrane can be introduced directly into the sample, regardless of the complexity of the sample. However, the extraction efficiency can be substantially improved when a systematic method development strategy is followed to optimize a number of factors that directly impact on the overall extraction efficiency of the FPSE membrane. The factors are presented in Figure 2 with their relative significance. As such, an analyst may decide which factor(s) should be given more attention during the method development exercises. The factors include:
FPSE method development exercises can be carried out using a conventional one-factor-at-a-time (One FAT) approach or using a chemometric design of experiment approach. The later approach is the more scientific and green approach, as it provides deep insight about the overall extraction process and sheds light as to whether different factors interact with each other or not. A screening design can be carried out to select factors with the most influence on the overall extraction efficiency. Subsequently, a response surface model (RSM) design can be employed to find the optimum levels of the most influential factors.
7. Applications
All developed methodologies reported in the literature since 2014 are briefly described below, showing the wide range of applicability of fabric phase sorptive extraction in terms of sample matrix and analyte diversity.
Many research groups all over the world have adopted this innovative sample preparation approach and have developed new analytical strategies to deal with significant analytical problems encountered in virtually all facets of analytical fields.
Kumar et al. in 2014 were the first group to implement fabric phase sorptive extraction (FPSE) in the development of a simple, fast, and sensitive analytical method using a sol–gel poly(tetrahydrofuran) (sol–gel PTHF) coated FPSE membrane for the quantification of endocrine disrupting chemicals (EDCs), including 17α-ethinyl estradiol (EE2), β-estradiol (E2) and bisphenol A (BPA). Analysis was performed by high performance liquid chromatography with fluorescence detection (HPLC-FLD). In their work, the authors have investigated and optimized various factors that influence the efficiency of FPSE technique. The developed method was applied successfully for the analysis of the examined estrogen molecules in urine and various kinds of aqueous samples with good reported recoveries, i.e., 96–98% for drinking water, 94–95% for ground water, 92–94% for river water, and 90–91% for urine samples, while lower detection limits of BPA, E2, and EE2 over previously reported methods were achieved within the range of 20 to 42 pg/mL. Linearity, precision, and accuracy results proved that the developed method is rapid, precise, reproducible, and sensitive for the determination of estrogens in urine and aqueous samples
[9].
A year later, in 2015, Roldán-Pijuán et al.
[10] presented for the first time a novel technique: the approach of stir fabric phase sorptive extraction (SFPSE), which integrates sol–gel hybrid organic–inorganic coated fabric phase sorptive extraction media with a magnetic stirring mechanism. Two flexible fabric substrates, namely cellulose and polyester, were utilized as the host matrix for three different sorbents, e.g., sol–gel poly(tetrahydrofuran) (sol–gel PTHF), sol–gel poly(ethylene glycol) (sol–gel PEG), and sol–gel poly(dimethyldiphenylsiloxane) (sol–gel PDMDPS). Triazine herbicides were selected as model compounds to evaluate the operational performance of this unique microextraction device. The factors affecting the extraction efficiency of SFPSE have been investigated, and the optimal extraction conditions using sol–gel PEG coated SFPSE device in combination with UPLC-DAD yielded limits of quantification (LOQs) for the seven triazine herbicides in the range of 0.26–1.50 μg/L, while the hyphenation with LC-MS/MS allowed the improvement of the method sensitivity to the range of 0.015 μg/L to 0.026 μg/L. Enrichment factors between 444 and 1411 were achieved. The developed method was finally applied for the determination of selected triazine herbicides from three river water samples. Relative recoveries of the target analytes, in the range of 75% to 126%, were found to be satisfactory, while absolute extraction recoveries were in the range of 22.2–70.5%.
One year later, in 2016, Anthemidis et al.
[11] developed a novel flow injection fabric disk sorptive extraction (FI-FDSE) system for the automated determination of trace metals. The platform was based on a mini-column packed with sol–gel coated fabric membrane in the form of disks, incorporated into an on-line solid-phase extraction system, coupled with flame atomic absorption spectrometry (FAAS). This configuration resulted in high loading flow rates and shorter analytical cycles due to the minor observed backpressure. The potentials of this technique were demonstrated for trace lead and cadmium determination in environmental water samples. Various sol–gel coated FPSE media were investigated. The on-line formed complex of metal with ammonium pyrrolidine dithiocarbamate (APDC) was retained onto the fabric surface. Among the examined sol–gel coated FPSE membranes, sol–gel PDMDPS coated membrane provided the best extraction sensitivity and excellent reproducibility due to its hydrophobic nature similar to that of metal-APDC complex. The analytes were subsequently eluted by methyl isobutyl ketone (MIBK) prior to atomization. Optimum parameters included 90 s preconcentration time, with a sampling frequency of 30 h
−1, and thus enrichment factors of 140 and 38 and detection limits of 1.8 and 0.4 μg L
−1 were achieved for lead and cadmium.
Alcudia Leon et al. in 2017
[12] presented a novel sampling device that integrates air sampling and preconcentration based on fabric phase sorptive extraction principles. The determination of the main components of the sexual pheromone of
Tuta absoluta [(3E,8Z,11Z)-tetradecatrien-1-yl acetate and (3E,8Z)-tetradecadien-1-yl acetate] traces in environmental air in tomato crops has been selected as a model system. A laboratory-built unit made up of commercial brass elements as a holder of the sol–gel coated fabric extracting phase was designed and optimized. The unit proved to efficiently work under sampling and analysis modes which eliminated any need for sorptive phase manipulation prior to instrumental analysis. In the sampling mode, the unit is connected to a sampling pump to pass the air through the sorptive phase under controlled flowrate. In the analysis mode, the unit is placed in the gas chromatograph autosampler without any instrumental modification, thus eliminating the risk of cross contamination between sampling and analysis. The limits of detection for both compounds resulted to be 1.6 μg and 0.8 μg.
Yang et al. in 2018
[13] proposed a green, simple, inexpensive, and sensitive ionic liquid immobilized fabric phase sorptive extraction method coupled with high performance liquid chromatography for the rapid screening and simultaneous determination of four fungicides (azoxystrobin, chlorothalonil, cyprodinil, and trifloxystrobin) residues in tea infusions. The optimum conditions were found to be 10% [HIMIM]NTf
2 as coating solution, 2 min vortex time, 500 μL acetonitrile as dispersive solvent, and 2 min desorption time. Under these conditions, the proposed technique was applied to detect fungicides from real tea water samples with satisfactory results.
Kaur et al. in 2019
[14] combined FPSE with gas chromatography mass spectrometry for the rapid extraction and determination of nineteen organochlorine pesticides in various fruit juices and water samples. The extraction approach was optimized in terms of sorbent chemistry, extraction time, stirring speed, type and volume of back-extraction solvent, and back-extraction time. Optimum conditions yielded limits of detection in a range of 0.007–0.032 ng/mL. The relative recoveries obtained by spiking organochlorine pesticides in water and selected juice samples were in the range of 91.56–99.83%. Sol–gel poly(ethylene glycol)-poly(propylene glycol)-poly(ethylene glycol) was proved to be the best sorbent for the extraction and preconcentration of organochlorine pesticides in aqueous and fruit juice samples prior to analysis with gas chromatography mass spectrometry.
Tartaglia et al. in 2019
[15] reported on the performance comparison between the exhaustive and equilibrium extraction using classical Avantor C18 solid phase extraction (SPE) sorbent, hydrophilic-lipophilic balance (HLB) SPE sorbent, Sep-Pak C18 SPE sorbent, novel sol–gel Carbowax 20M (sol–gel CW 20M) SPE sorbent, and sol–gel CW 20M coated fabric phase sorptive extraction (FPSE) media for the extraction of three inflammatory bowel disease (IBD) drugs. Both the commercial SPE phases and in-house synthesized sol–gel CW 20M SPE phases were loaded into SPE cartridges and the extractions were carried out under an exhaustive extraction mode, while FPSE was carried out under an equilibrium extraction mode. The method was validated in compliance with international guidelines for the bioanalytical method validation. Novel in-house synthesized and loaded sol–gel CW 20M SPE sorbent cartridges were characterized in terms of their extraction capability, breakthrough volume, retention volume, hold-up volume, number of the theoretical plate, and the retention factor.
The performance of FPSE and SPE techniques was evaluated by comparing the breakthrough volume and enrichment factors. The authors found that for the examined analytes, SPE showed the highest enrichment factors; consequently, this method is more suitable for samples with low analytes concentration.
Perez Mayan in 2019
[16] investigated the use of FPSE for the extraction and preconcentration of ultra-trace level residues of fungicides (19 compounds) and insecticides (3 species) in wine samples. Subsequently, the preconcentrated analytes were determined using ultra-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS). Experimental extraction parameters affecting the efficiency and repeatability of the extraction were optimized. Optimized conditions included cellulose fabric coated with a sol–gel polyethylene glycol sorbent and back extraction using ACN-MeOH (80:20
v/
v) mixture. Limits of quantification (LOQs) ranged between 0.03 and 0.3 ng mL
−1. Relative recoveries ranged from 77 ± 6% to 118 ± 4%, and from 87 ± 4% to 121 ± 6% for red and white wines, respectively. The applicability of the method was proved for commercial wines.
In 2019, Lioupi et al.
[17] developed and validated an innovative fabric phase sorptive extraction high-performance liquid chromatography–diode array detection (FPSE-HPLC-DAD) method for the extraction of five common antidepressants (venlafaxine, paroxetine, fluoxetine, amitriptyline, clomipramine) in human urine samples. The extraction protocol was optimized with regards to the extraction main parameters. Sol–gel graphene sorbent, coated on cellulose FPSE media, were the most efficient among other with different polarities using CH
3OH:CH
3CN (50:50
v/
v) for back-extraction. The absolute recovery values were 25.5% for venlafaxine, 33.9% for paroxetine, 67.0% for fluoxetine, 43.0% for amitriptyline, and 29.0% for clomipramine, while relative recoveries were higher than 90%. The developed method provides satisfactory limit of detection 0.15 ng/μL.
Locatelli in 2019
[18] proposed a fabric phase sorptive extraction based method prior to high-performance liquid chromatography–photodiode array detection (FPSE-HPLC-PDA) for the simultaneous extraction and analysis of six benzophenone derivative UV filters, including benzophenone (BZ), 5-benzoyl-4-hydroxy-methoxybenzenesulfonic acid (BP-4), bis(4-hydroxyphenyl)methanone (4-DHB), bis(2,4-dihydroxyphenyl)methanone (BP-2), (2,4-dihydroxybenzophenone) (BP-1), and 2,2′-dihydroxy-4-methoxybenzophenone (DHMB) in human whole blood, plasma, and urine samples. The limit of quantification was found to be 0.1 μg/mL. This new approach shows promising results with high potential for direct adaptation as a rapid, robust, and green analytical tool for several applications, e.g., in the current sample preparation practices used in many bioanalytical fields including pharmacokinetics (PK), pharmacodynamics (PD), therapeutic drug monitoring (TDM), clinical and forensic toxicology, disease diagnosis, and drug discovery. Optimized conditions included the use of sol–gel CW
®20M FPSE membrane with a 20:80 (%
v:v) mixture of phosphate buffer 40 mM at pH 3 and methanol.
Taraboletti et al. in 2019
[19] reported a metabolomics workflow using a mass spectrometry-compatible fabric phase sorptive extraction (FPSE) technique implementing a matrix coated with sol–gel poly(caprolactone-b-dimethylsiloxane-b-caprolactone) that binds both polar and nonpolar metabolites in whole blood, eliminating serum processing steps. FPSE preparation technique combined with liquid chromatography–mass spectrometry can distinguish radiation exposure markers such as taurine, carnitine, arachidonic acid, α-linolenic acid, and oleic acid found 24 h after 8 Gy irradiation. These findings suggest that the FPSE approach could work in future technology to triage irradiated individuals accurately, via biomarker screening, by providing a novel method to stabilize biofluids between collection and sample analysis.
Alampanos et al. in 2019
[20] proposed an environmentally friendly method by making use of high-performance liquid chromatography and photo-diode array detection (HPLC-PDA) for the determination of four penicillin antibiotics residues (benzylpenicillin, cloxacillin, dicloxacillin, and oxacillin) in human blood serum after FPSE. Solvent evaporation and reconstitution steps, which are considered to be rather time-consuming, were eradicated successfully from the sample preparation workflow, organic solvent consumption was brought to a minimum, while protein precipitation was assessed as impractical. Thus, the proposed method met all green analytical chemistry (GAC) criteria. The microextraction device was characterized by high chemical and solvent stability owing to the strong chemical bonds formed between the sol–gel sorbent and the substrate. Therefore, any organic solvent/solvent mixture can serve as the eluent/back-extraction solvent. The authors, after optimization of FPSE experimental parameters, propose sol–gel poly(tetrahydrofuran) coated FPSE membrane as the optimum extraction sensitivity for the selected penicillin antibiotics, after back-extraction using 90:10
v/
v acetonitrile and ammonium acetate (0.01M). For all four penicillin antibiotics, the limit of detection was 0.15 ng/μL.
Zilfidou et al. in 2019
[21] applied FPSE for the simple and rapid simultaneous extraction of five common antidepressant drug residues (venlafaxine, paroxetine, fluoxetine, amitriptyline, and clomipramine) from human blood serum. Elimination of protein precipitation step and minimized solvent consumption led to a sample preparation workflow compliant with the principles of green analytical chemistry (GAC). Among all the membrane examined, sol–gel polycaprolactone-dimethylsiloxane-polycaprolactone coated polyester substrate presented optimum extraction efficiency and was found to be reusable for at least 30 times. Back-extraction was achieved by methanol: acetonitrile (50:50
v/
v). The limit of detection was found at 0.15 ng μL
−1, while good absolute recoveries (9.4–88.1%) were obtained.
Tartaglia et al. in 2019
[22] described an FPSE based method for the simultaneous determination of seven paraben residues including methyl paraben (MPB), ethyl paraben (EPB), propyl paraben (PPB), isopropyl paraben (iPPB), butyl paraben (BPB), isobutyl paraben (iBPB), and benzyl paraben (BzPB) in human whole blood, plasma and urine, prior to high-performance liquid chromatography (HPLC) coupled with photo diode array detector (PDA) analysis. The analytical method has been validated according to the international guidelines.
The performance of the analytical method was evaluated on real biological samples. The proposed innovative method allows simultaneous analysis of seven paraben residues in three different biological matrices, including whole blood, plasma, and urine, and therefore it is easily applicable to monitor these substances in different biological samples. Furthermore, the extraction technique used in this work is fast, easy to use, and in accordance with the modern green analytical chemistry (GAC) principles. Sol–gel CW 20M FPSE media and back-extraction with methanol provided the best recovery rates.
Kaur et al. in 2019
[14] combined FPSE with gas chromatography–mass spectrometry for the rapid extraction and determination of nineteen organochlorine pesticides in various fruit juices and water samples. FPSE efficiency was optimized in terms of sorbent chemistry, extraction time, stirring speed, type and volume of back-extraction solvent, and back-extraction time. Under optimum conditions, the limits of detection were obtained in a range of 0.007–0.032 ng/mL. The relative recoveries obtained by spiking organochlorine pesticides in water and selected juice samples were in the range of 91.56–99.83%. The sorbent sol–gel poly(ethylene glycol)-poly(propylene glycol)-poly(ethylene glycol) was applied for the extraction and preconcentration of organochlorine pesticides in aqueous and fruit juice samples prior to analysis with gas chromatography–mass spectrometry.
Otoukesh et al. in 2019
[23] proposed a fabric phase sorptive extraction (FPSE) for the enrichment of acrylate compounds coming from acrylic adhesives used commonly for sticking the paper labels on polyethylene terephthalate (PET) bottles, and therefore they may exist in recycled polyethylene terephthalate (rPET) in different food simulants: simulant A (ethanol 10%), simulant B (acetic acid 3%), and simulant C (ethanol 20%), and their respective extracts by ultra-high-performance liquid chromatography with mass spectrometric detection (UPLC-MS). Four acrylates were studied: ethylene glycol dimethacrylate (EGDM), pentaerythritol triacrylate (PETA), triethylene glycol diacrylate (TEGDA), and trimethylolpropane triacrylate (TMPTA). Five different types of FPSE membrane coated with different sol–gel sorbents were studied, and finally sol–gel polyethylene glycol- polypropylene glycol-polyethylene glycol triblock copolymer (PEG-PPG-PEG) coated FPSE membrane was chosen for its satisfactory results combined with methanol for back-extraction since it provided an elution ability slightly higher than acetonitrile. Under the optimized conditions, the method provided limits of detection of the compounds in the range of (0.1–1.9 ng g
−1, 0.1–1.2 ng g
−1, 0.2–2.3 ng g
−1) in EtOH 10%, HAc 3%, and EtOH 20%, and the enrichment factor values (EFs) after applying N
2 were in the range of 11.1–25.0, 13.8–26.3, 8.3–21.9, in simulants A, B, and C, respectively. The optimized method was applied successfully to analyze thirteen types of recycled PET samples.
Mesa et al. in 2019
[8] developed a simple and sensitive analytical methodology for rapid screening and quantification of selected estrogenic endocrine disrupting chemicals including α-estradiol, hexestrol, estrone, 17α-ethinyl estradiol, diethylstilbestrol, and bisphenol A from intact milk using fabric phase sorptive extraction in combination with high-performance liquid chromatography coupled to ultraviolet detection/tandem mass spectrometry. The new approach eliminates protein precipitation and defatting step from the sample preparation workflow, while the error prone and time-consuming solvent evaporation and sample reconstitution steps have also been eliminated. Parameters which mostly affect the extraction efficiency of fabric phase sorptive extraction, including sorbent chemistry, sample volume, and extraction time, were optimized. The limit of detection values obtained in fabric phase sorptive extraction with high-performance liquid chromatography with ultraviolet detection ranged from 25.0 to 50.0 ng/mL.
Two sol–gel sorbent coatings were tested to determine the better sorbent coating for the selected EDCs, sol–gel PTHF, and sol–gel PDMS. Sol–gel PTHF was distinctly superior in extraction efficiencies for all compounds, with acetonitrile used for back extraction.
Lastovka et al. in 2019
[24] developed a method for the quantification of highly potent analgesic agent (2
R,4a
R,7
R,8a
R)-4,7-dimethyl-2-(thiophen-2-yl)octahydro-2
H-chromen-4-ol in rat whole blood and plasma using dried matrix spots (DMS) and fabric phase sorptive extraction (FPSE) techniques in combination with LC–MS/MS. The linearity was obtained in the range of 20–5000 ng/mL and 50–5000 ng/mL for plasma-FPSE and blood-FPSE experiments, respectively. The mean extraction recovery (%) was 26 for plasma-DMS, 25 for blood DMS, 38 for plasma-FPSE, and 31 for blood-FPSE.
A sol–gel PCAP-PDMS-PCAP sorbent-coated FPSE biofluid sampler FPSE blood sampler was compared to a DBS card and has been used under a different sampling and extraction mode (DBS card with direct spotting, and FPSE biofluid sampler with equilibrium extraction mode); both perform satisfactorily with different sample matrices. However, the FPSE biofluid sampler was found more selective in preparing an interferents-free sample for instrumental analysis. Due to the exploitation of high-performance sol–gel sorbent, the FPSE biofluid sampler has the potential to streamline the current practice of blood analysis.
Gulle et al. in 2019
[25] developed a fabric phase sorptive extraction (FPSE)-based sample preparation method for methyl paraben (MP), propyl paraben (PP), and butyl paraben (BP) in cosmetic and environmental samples, prior to high performance liquid chromatography–photodiode array (HPLC-PDA) detection. In the proposed method, MP, PP, and BP molecules were efficiently retained on a sol–gel Carbowax-20M sorbent-coated FPSE membrane when the matrix pH was adjusted to 5. Subsequently, the extracted analytes were desorbed from the FPSE membrane with methanol. Experimental conditions were studied to optimize variables such as pH, adsorption time, and desorption solvent. Using the optimal conditions, analytical parameters such as linearity ranges, detection limits, and preconcentration factors for each of the selected parabens were calculated from experimental data. The limit of detection (LOD) values for MP, PP, and BP were calculated as 2.85, 2.98, and 2.75 ng mL
−1, respectively. Finally, the developed method was applied to cosmetic and environmental samples.
Kaur et al. in 2019
[26] developed a high-efficiency and solvent minimized microextraction technique, fabric phase sorptive extraction followed by gas chromatography and mass spectrometry analysis for the rapid determination of four organophosphorus pesticides (terbufos, malathion, chlorpyrifos, and triazofos) in vegetable samples including beans, tomato, brinjal, and cabbage. The most important fabric phase sorptive extraction parameters were investigated and optimized. Under optimum experimental conditions, the limits of detection were found in the range of 0.033 to 0.136 ng/g. Three different sol–gel sorbent coated FPSE membranes were evaluated, including sol–gel Carbowax 20 M (sol–gel CW 20 M), sol–gel poly(tetrahydrofuran) (sol–gel PTHF), and sol–gel poly(dimethyl siloxane) (sol–gel PDMS). Sol–gel CW 20 M coated FPSE membrane was selected as the suitable FPSE membrane for the selected OPPs.
Sun et al. in 2019
[27] developed a new method which coupled FPSE with ion mobility spectrometry (IMS) for the rapid detection of polycyclic aromatic hydrocarbons (PAHs) in water present in the field. Polydimethylsiloxane (PDMS) was coated on the glass fiber cloth through a sol–gel reaction. After extracting the PAHs in water, the fabric coated PDMS could be directly put into the inlet of IMS instrument for thermal desorption. The PAHs were analyzed by the IMS instrument operated in the positive ion mode with a corona discharge (CD) ionization source. The primary parameters affecting extraction efficiency such as extraction time, extraction temperature, and ionic strength were investigated and optimized by using phenanthrene (Phe), benzo[a]anthracene (BaA), and benzo[a]pyrene (BaP) as model compounds. Under the optimal conditions, the FPSE-IMS detection limits were 5 ng mL
−1, 8 ng mL
−1, and 10 ng mL
−1, respectively. Satisfactory recoveries were obtained ranging from 80.5% to 100.5%, making the method of FPSE-IMS applicable for the monitoring the water quality on-site, and thus providing early warning in the field.
Kaur et al. in 2019
[28] developed and validated a rapid extraction and clean-up method using selective fabric phase sorptive extraction combined with gas chromatography and mass spectrometry for the determination of broad polarity spectrum emerging pollutants, ethyl paraben, butyl paraben, diethyl phthalate, dibutyl phthalate, lidocaine, prilocaine, triclosan, and bisphenol A in various aqueous samples. Some important parameters of fabric phase sorptive extraction such as extraction time, matrix pH, stirring speed, type, and volume of desorption solvent were investigated and optimized. Under the optimum conditions, the limits of detection were in the range 0.009–0.021 ng/mL. Recoveries ranged from 93 to 99%. The developed method was applied for the determination of the emerging contaminants in tap water, municipal water, ground water, sewage water, and sludge water samples. Three different FPSE sorbent coatings were comparatively studied: sol–gel CW20M (polar), sol–gel PTHF (medium polar), and sol–gel C18 (nonpolar). Sol–gel CW20M-coated FPSE provided the optimum results.
The most recent application comes from Celeiro et al.
[29]. This research group in 2020 proposed a novel method based on fabric phase sorptive extraction (FPSE) followed by gas chromatography–tandem mass spectrometry (GC-MS/MS) for the simultaneous determination of 11 UV filters (ethylhexyl salicylate, benzyl salicylate, homosalate, benzophenone-3, isoamylmethoxycinnamate, 4-methylbenzylidenecamphor, methyl anthranilate, etocrylene, 2-ethylhexylmethoxycinnamate, 2-ethylhexyl p-dimethylaminobenzoate, and octocrylene), in natural and recreational waters. Different types and sizes of sol–gel coated FPSE membranes, sample volumes, extraction times, and types and volumes of desorption solvent were optimized. The optimal conditions involved the use of a (2.0 × 2.5) cm
2 FPSE device with PDMS-based coating for the extraction of 20 mL of water for 20 min. Back-extraction was performed by ethyl acetate. Recovery rates under optimum conditions were about 90%. LODs were at the low ng L
−1 in all cases. The proposed validated FPSE-GC-MS/MS method was applied to different real samples, including environmental water (lake, river, seawater) and recreational water (swimming pool).