A severe mismatch between the supply and demand of oxygen is the common sequela of all types of shock, which present a mortality of up to 80%. Various organs play a protective role in shock and contribute to whole-body homeostasis. The ever-increasing number of multidetector CT examinations in severely ill and sometimes unstable patients leads to more frequently encountered findings leading to imminent death, together called “hypovolemic shock complex”. Features on CT include dense opacification of the right heart and major systemic veins, venous layering of contrast material and blood, densely opacified parenchyma in the right hepatic lobe, decreased enhancement of the abdominal organ, a dense pulmonary artery, contrast pooling in dependent lungs, and contrast stasis in pulmonary veins. These findings are biomarkers and prognostic indicators of paramount importance which stratify risk and improve patient outcomes.
- cardiogenic shock
- computed tomography
- contrast layering
- venous pooling
- hypovolemia
- hypovolemic shock complex
1. Introduction
|
Shock |
||||
|---|---|---|---|---|
|
Types |
Pathogenesis |
Causes |
Pathophysiology |
Treatment Targets |
|
Cardiogenic (13%) |
Sudden impairment of myocardial performance |
|
A critical reduction of the heart’s pumping capacity, a reduced ejection fraction or impaired ventricular filling |
Remove the cardiac causes of the shock |
|
Hypovolemic (27%) |
Inadequate organ perfusion caused by loss of intravascular volume |
|
Inadequate organ perfusion caused by acute loss of intravascular volume, drop in cardiac preload to a critical level |
Intravascular volume replacement, endotracheal intubation |
|
Distributive (59%) |
Hypovolemia resulting from pathological redistribution of the absolute intravascular volume |
|
Loss of regulation of vascular tone and/or disordered permeability of the vascular system |
Support circulation by infusion of balanced solutions, administration of vasopressors and/or inotropic drugs, organ replacement therapy |
|
Obstructive (1%) |
RV-LV Preload ↓ RV-LV Afterload ↑ Obstruction of the great vessels or the heart |
|
Intravasal/Intraluminal (e.g., PE, Leriche S., AD) Extravasal/extraluminal (e.g., Tension PNX, Tamponade) |
Immediate causal treatment (e.g., thrombolysis, thoracic or pericardial drainage; surgical embolectomy) |
2. Multidetector CT (MDCT) Technique
3. CT Patterns
- (a)
-
In cases of hemodynamic stability, IV CM into an upper limb vein is delivered to the right atrium via the superior vena cava (SVC), and is then pumped via the right ventricle to the pulmonary arteries. Contrast subsequently returns via the pulmonary veins to the left-side cardiac chambers before reaching systemic circulation [39]. As it undergoes first pass circulation and re-circulation, the contrast bolus gradually mixes with the blood pool, leading to dilution while moving downstream from the injection site. Due to its small molecular size, iodinated CM exhibits high diffusibility, readily redistributing from the intravascular space to organic interstitial spaces [39][40]. This may be called the “physiological” pattern and can correspond to an early compensatory stage of shock. Particularly in these patients without advanced shock symptoms, an image-based morphological indicator promises information about the identification of patients “at high risk”.
- (b)
-
In a state of advanced hemodynamic instability, many homeostatic mechanisms try to maintain arterial pressure and adequate tissue perfusion to critical organs, such as the brain and heart, by reflex stimulation of the sympathetic nervous system, elevated levels of angiotensin II, adrenaline, and noradrenaline, and vasoconstriction (compensated shock). Carotid baroreceptors respond to decreased blood pressure by triggering increased sympathetic signaling and maintaining cardiac output (sympathetic “fight or flight” response). In cases of decompensated shock, when compensatory mechanisms falter and prior to the onset of death, the pumping action of the heart ceases, leading to a substantial decline in systemic arterial and venous pressures. Consequently, the arteriovenous pressure gradient diminishes [6][41][42]. This altered hemodynamic state results in stasis of CM in the venous system in the presence of the left chamber and arterial opacification, and of other infrequent and often unappreciated ominous MDCT vascular signs that represent a true hypovolemic state and must be recognized early by the radiological staff to improve survival [24][43][44][45][46][47][48]. This may be called the “venous CM pooling and layering” pattern, indicating that compensatory mechanisms are becoming insufficient and the patient must receive immediate treatment.
- (c)
-
In irreversible end-organ dysfunction, injected IV CM circulation is supported only by the pressure applied by the automated power injector and the density of contrast material. Circulatory arrest leads to dense contrast pooling and layering in the SVC, IVC (inferior vena cava), and right heart chambers with non-opacified left heart chambers or arterial vessels (Figure 1) [43][45][49][50][51][52]. This may be called the “non-beating heart” pattern. Cardio-pulmonary aggressive resuscitation must immediately be initiated within the framework of a predetermined emergency plan.
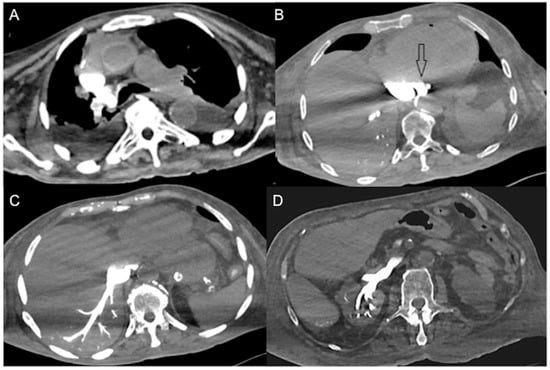
Figure 1. Non-beating heart in a 72-year-old man with sudden-onset severe dyspnea/shock and asystole during thoraco-abdominal CT. (A) CECT axial image shows dense contrast in the round superior vena cava, and reflux in the azygous arch; (B) contrast pooling and layering in the right atrium and IVC with retrograde opacification of coronary sinus (arrow). (C) CM fills the round inferior vena cava with hypostatic reflux into the hepatic veins, hemiazygos vein, partially splenic vein, and (D) right renal vein. Note no mixing of blood with CM and no opacification of the pulmonary arteries, aorta, and left cardiac chambers, suggestive of a non-beating heart. Prompt initiation of cardio-pulmonary resuscitation to restore circulation was useless. Autopsy: ruptured myocardial infarction.
4. CT-Updated HSC Findings as Diagnostic Biomarkers
5. CECT Findings/Biomarkers as Prognostic Indicators
|
CECT Findings |
Cardiogenic |
Distributive |
Hypovolemic |
Obstructive |
Outcome |
|---|---|---|---|---|---|
|
Small-caliber aorta |
~25% |
~28% |
~30% |
~35% |
poor |
|
Slit/flattened cava |
~70% |
~55% |
~77% |
~50% |
very poor |
|
Halo sign IVC |
~70% |
~65% |
~75% |
NA |
poor |
|
Narrow SMA/V |
NA |
NA |
NA |
NA |
NA |
|
Lack of left AV enhancement |
~65% |
~35% |
~55% |
~20–50% |
very poor |
|
CM vascular layering |
~75% |
NA |
~65% |
~70% |
very poor |
|
Hot-spot sign |
NA |
NA |
NA |
NA |
NA |
|
Periportal halo |
~60% |
NA |
~40% |
NA |
NA |
|
Ongoing hemorrhage |
10% |
15% |
65% |
25% |
poor |
|
Shock Thyroid |
NA |
NA |
NA |
NA |
very poor |
|
Shock Lungs |
NA |
NA |
NA |
NA |
NA |
|
Shock Bowel |
~55% |
~50% |
~70% |
~40% |
poor |
|
Shock Spleen |
~40% |
~50% |
~50% |
~25% |
poor |
|
Liver altered density |
~85% |
~55% |
~57% |
~45% |
poor |
|
Shock gallbladder |
20–30% |
~12% |
13–35% |
~9% |
poor |
|
Shock pancreas |
~35% |
~55% |
~45% |
~35% |
very poor |
|
Shock Stomach |
NA |
NA |
NA |
NA |
NA |
|
Shock Kidneys |
~55% |
~50% |
~60% |
~40% |
poor |
|
Shock Adrenals |
~60% |
~65% |
~55% |
~50% |
poor |
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13132304
References
- Haseer Koya, H.; Paul, M. Shock. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531492/ (accessed on 25 July 2022).
- Zotzmann, V.; Rottmann, F.A.; Müller-Pelzer, K.; Bode, C.; Wengenmayer, T.; Staudacher, D.L. Obstructive Shock, from Diagnosis to Treatment. Rev. Cardiovasc. Med. 2022, 23, 248.
- Bonanno, F.G. Clinical pathology of the shock syndromes. J. Emerg. Trauma Shock 2011, 4, 233–243.
- Kumar, A.; Parrillo, J.E. Shock: Classification, Pathophysiology, and Approach to Management. Critical Care Medicine, 3rd ed.; Elsevier Inc.: Philadelphia, PA, USA, 2008; pp. 379–422.
- Vincent, J.L.; De Backer, D. Circulatory shock. N. Engl. J. Med. 2013, 369, 1726–1734.
- Standl, T.; Annecke, T.; Cascorbi, I.; Heller, A.R.; Sabashnikov, A.; Teske, W. The Nomenclature, Definition and Distinction of Types of Shock. Dtsch. Arztebl. Int. 2018, 115, 757–768.
- Vahdatpour, C.; Collins, D.; Goldberg, S. Cardiogenic Shock. J. Am. Heart Assoc. 2019, 8, e011991.
- Lambden, S.; Creagh-Brown, B.C.; Hunt, J.; Summers, C.; Forni, L.G. Definitions and pathophysiology of vasoplegic shock. Crit. Care 2018, 22, 174.
- Volski, A.J.; Ackerman, D. Neurogenic Shock . In Clinical Management of Shock—The Science and Art of Physiological Restoration; IntechOpen: London, UK, 2020.
- Dugar, S.; Choudhary, C.; Duggal, A. Sepsis and septic shock: Guideline-based management. Clevel. Clin. J. Med. 2020, 87, 53–64.
- Leech, C.; Turner, J. Shock in Trauma. Emerg. Med. Clin. N. Am. 2023, 41, 1–17.
- Kislitsina, O.N.; Rich, J.D.; Wilcox, J.E.; Pham, D.T.; Churyla, A.; Vorovich, E.B.; Ghafourian, K.; Yancy, C.W. Shock—Classification and Pathophysiological Principles of Therapeutics. Curr. Cardiol. Rev. 2019, 15, 102–113.
- Kim, Y.J.; Kim, J.S.; Cho, S.H.; Bae, J.I.; Sohn, C.H.; Lee, Y.S.; Lee, J.H.; Lim, K.S.; Kim, W.Y. Characteristics of computed tomography in hemodynamically unstable blunt trauma patients: Experience at a tertiary care center. Medicine 2017, 96, e9168.
- Valente, T.; Sica, G.; Bocchini, G.; Romano, F.; Lassandro, F.; Rea, G.; Muto, E.; Pinto, A.; Iacobellis, F.; Crivelli, P.; et al. MDCT Imaging of Non-Traumatic Thoracic Aortic Emergencies and Its Impact on Diagnosis and Management-A Reappraisal. Tomography 2022, 13, 200–228.
- Taylor, G.A.; Fallat, M.E.; Eichelberger, M.R. Hypovolemic shock in children: Abdominal CT manifestations. Radiology 1987, 164, 479–481.
- Sivit, C.J.; Taylor, G.A.; Bulas, D.I.; Kushner, D.C.; Potter, B.M.; Eichelberger, M.R. Posttraumatic shock in children: CT findings associated with hemodynamic instability. Radiology 1992, 182, 723–726.
- Rotondo, A.; Catalano, O.; Grassi, R.; Scialpi, M.; Angelelli, G. Thoracic CT findings at Hypovolemic shock. Acta Radiol. 1998, 39, 400–404.
- Ryan, M.F.; Hamilton, P.A.; Sarrazin, J.; Chu, P.; Benjaminov, O.; Lam, K. The halo sign and peripancreatic fluid: Useful CT signs of hypovolaemic shock complex in adults. Clin. Radiol. 2005, 60, 599–607.
- Tarrant, A.M.; Ryan, M.F.; Hamilton, P.A.; Benjaminov, O. A pictorial review of hypovolaemic shock in adults. Br. J. Radiol. 2008, 81, 252–257.
- Lubner, M.; Demertzis, J.; Lee, J.Y.; Appleton, C.M.; Bhalla, S.; Menias, C.O. CT evaluation of shock viscera: A pictorial review. Emerg. Radiol. 2008, 15, 1–11.
- Ames, J.T.; Federle, M.P. CT hypotension complex (shock bowel) is not always due to traumatic hypovolemic shock. Am. J. Roentgenol. 2009, 192, W230–W235.
- Prasad, K.R.; Kumar, A.; Gamanagatti, S.; Chandrashekhara, S.H. CT in post-traumatic hypoperfusion complex—A pictorial review. Emerg. Radiol. 2011, 18, 139–143.
- Kanki, A.; Ito, K.; Tamada, T.; Higashi, H.; Sato, T.; Tanimoto, D.; Higaki, A. Dynamic contrast-enhanced CT of the abdomen to predict clinical prognosis in patients with hypovolemic shock. Am. J. Roentgenol. 2011, 197, W980–W984.
- Bagheri, S.M.; Taheri, M.S.; Pourghorban, R.; Shabani, M. Computed tomographic imaging features of sudden cardiac arrest and impending cardiogenic shock. J. Comput. Assist. Tomogr. 2012, 36, 291–294.
- Wang, J.; Liang, T.; Louis, L.; Nicolaou, S.; McLaughlin, P.D. Hypovolemic shock complex in the trauma setting: A pictorial review. Can. Assoc. Radiol. J. 2013, 64, 156–163.
- Higashi, H.; Kanki, A.; Watanabe, S.; Yamamoto, A.; Noda, Y.; Yasokawa, K.; Higaki, A.; Tamada, T.; Ito, K. Traumatic hypovolemic shock revisited: The spectrum of contrast-enhanced abdominal computed tomography findings and clinical implications for its management. J. Radiol. 2014, 32, 579–584.
- Smithson, L.; Morrell, J.; Kowalik, U.; Flynn, W.; Guo, W. Correlation of computed tomographic signs of hypoperfusion and clinical hypoperfusion in adult blunt trauma patients. J. Trauma Acute Care Surg. 2015, 78, 1162–1167.
- Anand, T.; vanSonnenberg, E.; Gadani, K.; Skinner, R. A snapshot of circulation failure following acute traumatic injury: The expansion of computed tomography beyond injury diagnosis. Injury 2016, 47, 50–52.
- Yüce, İ. CT Hypoperfusion Complex: Emergency CT Results During One Year. Eurasian J. Emerg. Med. 2016, 15, 136–138.
- Elst, J.; Ghijselings, I.E.; Zuidema, W.P.; Berger, F.H. Signs of post-traumatic hypovolemia on abdominal CT and their clinical importance: A systematic review. Eur. J. Radiol. 2020, 124, 108800.
- Di Serafino, M.; Viscardi, D.; Iacobellis, F.; Giugliano, L.; Barbuto, L.; Oliva, G.; Ronza, R.; Borzelli, A.; Raucci, A.; Pezzullo, F.; et al. Computed tomography imaging of septic shock. Beyond the cause: The “CT hypoperfusion complex”. A pictorial essay. Insights Imaging 2021, 12, 70.
- Cohen, I.; Tau, N.; Lekach, R.; Ironi, A.; Kraus, M.; Guranda, L. CT signs of hypovolemic shock complex in patients with non-traumatic shock. Abdom. Radiol. 2023, 48, 229–235.
- Alexander, L.F.; Hanna, T.N.; Legout, J.D.; Roda, M.S.; Cernigliaro, J.G.; Mittal, P.K.; Harri, P.A. Multidetector CT findings in the abdomen and pelvis after damage control surgery for acute traumatic injuries. Radiographics 2019, 39, 1183–1202.
- Wirth, S.; Hebebrand, J.; Basilico, R.; Berger, F.H.; Blanco, A.; Calli, C.; Dumba, M.; Linsenmaier, U.; Mück, F.; Nieboer, K.H.; et al. European Society of Emergency Radiology: Guideline on radiological polytrauma imaging and service (short version). Insights Imaging 2020, 11, 135.
- Pescatori, L.C.; Brambati, M.; Messina, C.; Mauri, G.; Di Leo, G.; Silvestri, E.; Sardanelli, F.; Sconfienza, L.M. Clinical impact of computed tomography in the emergency department in nontraumatic chest and abdominal conditions. Emerg. Radiol. 2018, 25, 393–398.
- Iacobellis, F.; Abu-Omar, A.; Crivelli, P.; Galluzzo, M.; Danzi, R.; Trinci, M.; Dell’Aversano Orabona, G.; Conti, M.; Romano, L.; Scaglione, M. Current Standards for and Clinical Impact of Emergency Radiology in Major Trauma. Int. J. Environ. Res. Public Health 2022, 19, 539.
- Flammia, F.; Chiti, G.; Trinci, M.; Danti, G.; Cozzi, D.; Grassi, R.; Palumbo, P.; Bruno, F.; Agostini, A.; Fusco, R.; et al. Optimization of CT protocol in polytrauma patients: An update. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2543–2555.
- Harrieder, A.; Geyer, L.L.; Körner, M.; Deak, Z.; Wirth, S.; Reiser, M.; Linsenmaier, U. Evaluation der Strahlendosis bei Polytrauma-CT-Untersuchungen eines 64-Zeilen-CT im Vergleich zur 4-Zeilen-CT. RöFo 2012, 184, 443–449.
- Bae, K.T. Intravenous contrast medium administration and scan timing at CT: Considerations and approaches. Radiology 2010, 256, 32–61.
- Fleischmann, D. CT angiography: Injection and acquisition technique. Radiol. Clin. N. Am. 2010, 48, 237–247.
- Reynolds, H.R.; Hochman, J.S. Cardiogenic Shock. Circulation 2008, 117, 686–697.
- Abassi, Z.; Khoury, E.E.; Karram, T.; Aronson, D. Edema formation in congestive heart failure and the underlying mechanisms. Front. Cardiovasc. Med. 2022, 27, 933215.
- Tsai, P.P.; Chen, J.H.; Huang, J.L.; Shen, W.C. Dependent pooling. Am. J. Roentgenol. 2002, 178, 1095–1099.
- Roth, C.; Sneider, M.; Bogot, N.; Todd, M.; Cronin, P. Dependent venous contrast pooling and layering: A sign of imminent cardiogenic shock. Am. J. Roentgenol. 2006, 186, 1116–1119.
- Wu, C.; Lee, R.; Wu, M.; Chang, C.; Wu, C. A blood-contrast level: A sign of cardiac arrest. J. Radiol. Sci. 2015, 40, 133–135.
- El Hasbani, G.; Lopez, E.O.; Castro, A.R.R.; Abouzeid, B.; Assaker, R.; Gamarra, J.V.; Khan, A.; Saeed, Y.; Al Husayni, H. Cardiac arrest identified by a chest CT scan in a patient with normal telemetry findings. Radiol. Case Rep. 2019, 14, 652–655.
- Sullivan, I.W.; Hota, P.; Dako, F.; Hajdinaj, S.; Davila, B. Dependent layering of venous refluxed contrast: A sign of critically low cardiac output. Radiol. Case Rep. 2019, 14, 230–234.
- Lee, Y.H.; Chen, J.; Chen, P.A.; Sun, J.T.; Kang, B.H.; Chu, S.E.; Fan, C.M.; Tsai, K.C.; Sim, S.S. Contrast Agent Pooling (C.A.P.) sign and imminent cardiac arrest: A retrospective study. BMC Emerg. Med. 2022, 22, 77.
- Shiotani, S.; Kohno, M.; Ohashi, N.; Yamazaki, K.; Itai, Y. Postmortem intravascular high density fluid level (hypostasis): CT findings. J. Comput. Assist. Tomogr. 2002, 26, 892–893.
- Singh, A.K.; Gervais, D.; Mueller, P.; Shirkhoda, A.; Sagar, P.; Mccarroll, K. Cardiac arrest: Abdominal CT imaging features. Abdom. Imaging 2004, 29, 177–179.
- Offiah, C.E.; Dean, J. Post-mortem CT and MRI: Appropriate post-mortem imaging appearances and changes related to cardiopulmonary resuscitation. Br. J. Rad. 2016, 89, 20150851.
- Sinha, A.; Bhatia, V.; Debi, U.; Singh, L.; Bhalla, A.; Sandhu, M. Imaging in Circulatory Arrest: Lessons to be Learned. J. Clin. Imaging Sci. 2019, 24, 44.
- Alabousi, M.; Mellnick, V.M.; Kashef Al-Ghetaa, R.; Patlas, M.N. Imaging of blunt bowel and mesenteric injuries: Current status. Eur. J. Radiol. 2020, 125, 108894.
- Shin, M.S.; Berland, L.L.; Ho, K.J. Small aorta: CT detection and clinical significance. J. Comput. Assist. Tomogr. 1990, 14, 102–103.
- Jonker, F.H.; Verhagen, H.J.; Mojibian, H.; Davis, K.A.; Moll, F.L.; Muhs, B.E. Aortic endograft sizing in trauma patients with hemodynamic instability. J. Vasc. Surg. 2010, 52, 39–44.
- Jonker, F.H.; Mojibian, H.; Schlosser, F.J.; Botta, D.M.; Indes, J.E.; Moll, F.L.; Muhs, B.E. The impact of hypovolaemic shock on the aortic diameter in a porcine model. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 564–571.
- Jeffrey, R.B.; Federle, M.P. The collapsed inferior vena cava: CT evidence of hypovolemia. Am. J. Roentgenol. 1988, 150, 431–432.
- Eisenstat, R.S.; Whitford, A.C.; Lane, M.J.; Katz, D.S. The “flat cava” sign revisited: What is its significance in patients without trauma? Am. J. Roentgenol. 2002, 178, 21–25.
- Matsumoto, S.; Sekine, K.; Yamazaki, M.; Sasao, K.; Funabiki, T.; Shimizu, M.; Yoshii, H.; Kishikawa, M.; Kitano, M. Predictive Value of a Flat Inferior Vena Cava on Initial Computed Tomography for Hemodynamic Deterioration in Patients with Blunt Torso Trauma. J. Trauma Inj. Infect. Crit. Care 2010, 69, 1398–1402.
- Johnson, J.J.; Garwe, T.; Albrecht, R.M.; Adeseye, A.; Bishop, D.; Fails, R.B.; Shepherd, D.W.; Lees, J.S. Initial inferior vena cava diameter on computed tomographic scan independently predicts mortality in severely injured trauma patients. J. Trauma Acute Care Surg. 2013, 74, 741–746.
- Nguyen, A.; Plurad, D.S.; Bricker, S.; Neville, A.; Bongard, F.; Putnam, B.; Kim, D.Y. Flat or fat? Inferior vena cava ratio is a marker for occult shock in trauma patients. J. Surg. Res. 2014, 192, 263–267.
- Kim, J.H.; Kim, W.Y.; Oh, J.; Kang, H.; Lim, T.H.; Ko, B.S. Association of inferior vena cava diameter ratio measured on computed tomography scans with the outcome of patients with septic shock. Medicine 2020, 99, e22880.
- Kim, D.W.; Yoo, H.S.; Kang, W.S. Flat Inferior Vena Cava on Computed Tomography for Predicting Shock and Mortality in Trauma: A Meta-Analysis. Diagnostics 2022, 12, 2972.
- Arslan, C.E.; Yeşilaras, M.; Atilla, Ö.D. Does Flattened IVC on CT Can Show Hypovolemia in Trauma Patients? Anatol. J. Emerg. Med. 2020, 3, 1–5.
- Wong, H.Y.; Lee, K.H. The IVC contrast level sign. Abdom. Radiol. 2017, 42, 2962–2963.
- Sueyoshi, E.; Imamura, T.; Sakamoto, I.; Uetani, M.; Matsuoka, Y. Contrast-fluid level in the inferior vena cava (IVC niveau sign) in patients with acute type A aortic dissection: Computed tomography findings during acute cardiac tamponade. J. Radiol. 2010, 28, 278–282.
- Verma, M.; Bhatia, V.; Singh, L.; Debi, U.; Sandhu, M. IVC contrast level: A sign of cardiovascular dysfunction. Oxf. Med. Case Rep. 2019, 1, omz068.
- Winzer, R.; Martin, R.; Baldus, J.C.; Heidrich, F.M.; Hoberück, S.; Hoffmann, R.T.; Fedders, D. Vascular changes of the superior mesenteric artery (SMA): A new component of the hypovolemic shock complex (HSC). Eur. J. Radiol. 2020, 133, 109370.
- Dickson, A.M. The focal hepatic hot spot sign. Radiology 2005, 237, 647–648.
- Hoang, V.T.; Vo, N.Q.; Trinh, C.T.; Nguyen, H.Q.; Chansomphou, V.; Le, T.B. The focal hepatic hot spot sign with lung cancer in computed tomography. Respirol. Case Rep. 2020, 8, e00671.
- Gunter, D.; Riaz, S.; Haider, E.A.; Rebello, R.; Patlas, M.N.; Alabousi, A. Hepatic perfusional changes on CT and MRI: A radiology primer. Abdom. Radiol. 2021, 46, 179–196.
- Koslin, D.B.; Stanley, R.J.; Berland, L.L.; Shin, M.S.; Dalton, S.C. Hepatic perivascular lymphedema: CT appearance. Am. J. Roentgenol. 1988, 150, 111–113.
- Lawson, T.L.; Thorsen, M.K.; Erickson, S.J.; Perret, R.S.; Quiroz, F.A.; Foley, W.D. Periportal halo: A CT sign of liver disease. Abdom. Imaging 1993, 18, 42–46.
- Barakat, F.; Kaisers, U.; Busch, T.; Donaubauer, B.; Hamm, B.; Röttgen, R. Periportal oedema of the liver-Correlation with clinical and paraclinical parameters in polytraumatic patients. Clin. Imaging 2009, 33, 39–43.
- Dressel-Böhm, S.; Richter, H.; Kircher, P.R.; Del Chicca, F. Hypoattenuating periportal halo on CT in a patient population can occur in presence of a variety of diseases. PLoS ONE 2022, 17, e0260436.
- Willmann, J.K.; Roos, J.E.; Platz, A.; Pfammatter, T.; Hilfiker, P.R.; Marincek, B.; Weishaupt, D. Multidetector CT: Detection of active hemorrhage in patients with blunt abdominal trauma. Am. J. Roentgenol. 2002, 179, 437–444.
- Kim, W.H.; Kim, M.S.; Kim, J.H.; Lee, K.H.; Lee, J.H. Shock Thyroid in a Patient with Septic Shock: A Case Report and Literature Review. J. Korean Soc. Radiol. 2021, 82, 1328–1333.
- Brochert, A.; Rafoth, J.B. Shock thyroid: A new manifestation of the hypovolemic shock complex in trauma patients. J. Comput. Assist. Tomogr. 2006, 30, 310–312.
- Han, D.H.; Ha, E.J.; Sun, J.S.; Jung, S.L. Remarkable CT features of shock thyroid in traumatic and non-traumatic patients. Emerg. Radiol. 2017, 24, 319–324.
- Ryu, H.M.; Yoo, J.Y.; Kim, S.J. Computed Tomographic Features of Lung Parenchyma Over Time after Cardiopulmonary Resuscitation. J. Korean Soc. Radiol. 2019, 80, 740–749.
- Mirvis, S.E.; Shanmuganathan, K.; Erb, R. Diffuse small-bowel ischemia in hypotensive adults after blunt trauma (shock bowel): CT findings and clinical significance. Am. J. Roentgenol. 1994, 163, 1375–1379.
- Rha, S.E.; Ha, H.K.; Lee, S.H.; Kim, J.H.; Kim, J.K.; Kim, J.H.; Kim, P.N.; Lee, M.G.; Auh, Y.H. CT and MR imaging findings of bowel ischemia from various primary causes. Radiographics 2000, 20, 29–42.
- Wittenberg, J.; Harisinghani, M.G.; Jhaveri, K.; Varghese, J.; Mueller, P.R. Algorithmic approach to CT diagnosis of the abnormal bowel wall. Radiographics 2002, 22, 1093–1107.
- Sugi, M.D.; Menias, C.O.; Lubner, M.G.; Bhalla, S.; Mellnick, V.M.; Kwon, M.H.; Katz, D.S. CT Findings of Acute Small-Bowel Entities. Radiographics 2018, 38, 1352–1369.
- Hiraiwa, H.; Okumura, T.; Sawamura, A.; Kondo, T.; Kazama, S.; Kimura, Y.; Shibata, N.; Arao, Y.; Oishi, H.; Kato, H.; et al. Spleen size improvement in advanced heart failure patients using a left ventricular assist device. Artif. Organs 2020, 44, 700–708.
- Kiguchi, T.; Higuchi, T.; Takahashi, N.; Shimokoshi, T.; Yamazaki, M.; Yoshimura, N.; Aoyama, H. CT measurement of splenic volume changes as a result of hypovolemic shock. J. Radiol. 2015, 33, 645–649.
- Enslow, M.S.; Preece, S.R.; Wildman-Tobriner, B.; Enslow, R.A.; Mazurowski, M.; Nelson, R.C. Splenic contraction: A new member of the hypovolemic shock complex. Abdom. Radiol. 2018, 43, 2375–2383.
- Hiraiwa, H.; Okumura, T.; Murohara, T. The cardiosplenic axis: The prognostic role of the spleen in heart failure. Heart Fail. Rev. 2022, 27, 2005–2015.
- Zha, A.; Vahidy, F.; Randhawa, J.; Parsha, K.; Bui, T.; Aronowski, J.; Savitz, S.I. Association between splenic contraction and the systemic inflammatory response after acute ischemic stroke varies with age and race. Transl. Stroke Res. 2018, 9, 484–492.
- Schagatay, E.; Lunde, A.; Nilsson, S.; Palm, O.; Lodin-Sundström, A. Spleen contraction elevates hemoglobin concentration at high altitude during rest and exercise. Eur. J. Appl. Physiol. 2020, 120, 2693–2704.
- Nishie, A.; Yoshimitsu, K.; Irie, H.; Tajima, T.; Asayama, Y.; Hirakawa, M.; Nakayama, T.; Kakihara, D.; Honda, H. Spectrum of hepatic surface enhancement on contrast-enhanced CT in various abdominal conditions. Clin. Imaging 2007, 31, 329–334.
- Wildman-Tobriner, B.; Enslow, M.S.; Nelson, R.C. Hepatic Heterogeneity and Attenuation on Contrast-Enhanced CT in Patients With the Hypovolemic Shock Complex: Objective Classification Using a Contemporary Cohort. Curr. Probl. Diagn. Radiol. 2019, 48, 224–228.
- Počepavičiūtė, K.; Pavilionė, R. CT hypoperfusion complex: How to recognize shock in the absence of profound hypotension. Radiol. Update 2021, 5, 13–20.
- Sivit, C.J.; Eichelberger, M.R.; Taylor, G.A. CT in children with rupture of the bowel caused by blunt trauma: Diagnostic efficacy and comparison with hypoperfusion complex. Am. J. Roentgenol. 1994, 163, 1195–1198.
- O’Hara, S.M.; Donnelly, L.F. Intense contrast enhancement of the adrenal glands: Another abdominal CT finding associated with hypoperfusion complex in children. Am. J. Roentgenol. 1999, 173, 995–997.
- Cheung, S.C.; Lee, R.; Tung, H.K.; Chan, F.L. Persistent adrenal enhancement may be the earliest CT sign of significant hypovolaemic shock. Clin. Radiol. 2003, 58, 315–318.
- Hrabak-Paar, M. Intense Adrenal Enhancement: A CT Feature of Cardiogenic Shock. Cardiovasc. Intervent. Radiol. 2016, 39, 296–298.
- Boos, J.; Schek, J.; Kropil, P.; Heusch, P.; Heinzler, N.; Antoch, G.; Lanzman, R.S. Contrast-enhanced computed tomography in intensive care unit patients with acute clinical deterioration: Impact of hyperattenuating adrenal glands. Can. Assoc. Radiol. J. 2017, 68, 21–26.
- Winzer, R.; Martin, R.; Kühn, J.P.; Baldus, J.C.; Seppelt, D.; Heidrich, F.M.; Hoberück, S.; Hoffmann, R.T.; Fedders, D. Adrenal glands enhancement in computed tomography as predictor of short-and intermediate term mortality in critically ill patients. Clin. Imaging 2021, 70, 56–60.
- Peng, Y.; Xie, Q.; Wang, H.; Lin, Z.; Zhang, F.; Zhou, X.; Guan, J. The hollow adrenal gland sign: A newly described enhancing pattern of the adrenal gland on dual-phase contrast-enhanced CT for predicting the prognosis of patients with septic shock. Eur. Radiol. 2019, 29, 5378–5385.
- Winzer, R.; Martin, R.; Kaiser, D.; Baldus, J.C.; Hoberück, S.; Hoffmann, R.T.; Fedders, D. Bilateral adrenal enhancement revised-adrenal-to-spleen ratio as an appropriate mortality predictor. Abdom. Radiol. 2021, 46, 2107–2114.
- Winzer, R.; Hoffmann, R.T.; Fedders, D. The Portal-Venous Enhancement Ratio of the Adrenal Glands and Spleen as a Short-Term Predictor of Mortality in Intensive Care Patients. Rofo 2022, 194, 1250–1257.
- Higashi, H.; Tamada, T.; Kanki, A.; Yamamoto, A.; Ito, K. Hypovolemic shock complex: Does the pancreatic perfusion increase or decrease at contrast-enhanced dynamic CT? Clin. Imaging 2014, 38, 31–34.
- Chaari, A.; Abdel Hakim, K.; Bousselmi, K.; Etman, M.; El Bahr, M.; El Saka, A.; Hamza, E.; Ismail, M.; Khalil, E.M.; Kauts, V.; et al. Pancreatic injury in patients with septic shock: A literature review. World J. Gastrointest. Oncol. 2016, 15, 526–531.
- Lomax, A.E.; Sharkey, K.A.; Furness, J.B. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol. Motil. 2010, 22, 7–18.
- Catalano, O.A.; Napolitano, M.; Vanzulli, A. Black kidney sign: A new computed tomographic finding associated with the hypoperfusion complex in children. J. Comput. Assist. Tomogr. 2005, 29, 484–486.
