A severe mismatch between the supply and demand of oxygen is the common sequela of all types of shock, which present a mortality of up to 80%. Various organs play a protective role in shock and contribute to whole-body homeostasis. The ever-increasing number of multidetector CT examinations in severely ill and sometimes unstable patients leads to more frequently encountered findings leading to imminent death, together called “hypovolemic shock complex”. Features on CT include dense opacification of the right heart and major systemic veins, venous layering of contrast material and blood, densely opacified parenchyma in the right hepatic lobe, decreased enhancement of the abdominal organ, a dense pulmonary artery, contrast pooling in dependent lungs, and contrast stasis in pulmonary veins. These findings are biomarkers and prognostic indicators of paramount importance which stratify risk and improve patient outcomes.
- cardiogenic shock
- computed tomography
- contrast layering
- venous pooling
- hypovolemia
- hypovolemic shock complex
1. Introduction
|
Shock |
||||
|---|---|---|---|---|
|
Types |
Pathogenesis |
Causes |
Pathophysiology |
Treatment Targets |
|
Cardiogenic (13%) |
Sudden impairment of myocardial performance |
|
A critical reduction of the heart’s pumping capacity, a reduced ejection fraction or impaired ventricular filling |
Remove the cardiac causes of the shock |
|
Hypovolemic (27%) |
Inadequate organ perfusion caused by loss of intravascular volume |
|
Inadequate organ perfusion caused by acute loss of intravascular volume, drop in cardiac preload to a critical level |
Intravascular volume replacement, endotracheal intubation |
|
Distributive (59%) |
Hypovolemia resulting from pathological redistribution of the absolute intravascular volume |
|
Loss of regulation of vascular tone and/or disordered permeability of the vascular system |
Support circulation by infusion of balanced solutions, administration of vasopressors and/or inotropic drugs, organ replacement therapy |
|
Obstructive (1%) |
RV-LV Preload ↓ RV-LV Afterload ↑ Obstruction of the great vessels or the heart |
|
Intravasal/Intraluminal (e.g., PE, Leriche S., AD) Extravasal/extraluminal (e.g., Tension PNX, Tamponade) |
Immediate causal treatment (e.g., thrombolysis, thoracic or pericardial drainage; surgical embolectomy) |
2. Multidetector CT (MDCT) Technique
3. CT Patterns
- (a)
-
In cases of hemodynamic stability, IV CM into an upper limb vein is delivered to the right atrium via the superior vena cava (SVC), and is then pumped via the right ventricle to the pulmonary arteries. Contrast subsequently returns via the pulmonary veins to the left-side cardiac chambers before reaching systemic circulation [39]. As it undergoes first pass circulation and re-circulation, the contrast bolus gradually mixes with the blood pool, leading to dilution while moving downstream from the injection site. Due to its small molecular size, iodinated CM exhibits high diffusibility, readily redistributing from the intravascular space to organic interstitial spaces [39,40]. This may be called the “physiological” pattern and can correspond to an early compensatory stage of shock. Particularly in these patients without advanced shock symptoms, an image-based morphological indicator promises information about the identification of patients “at high risk”.
- (b)
-
In a state of advanced hemodynamic instability, many homeostatic mechanisms try to maintain arterial pressure and adequate tissue perfusion to critical organs, such as the brain and heart, by reflex stimulation of the sympathetic nervous system, elevated levels of angiotensin II, adrenaline, and noradrenaline, and vasoconstriction (compensated shock). Carotid baroreceptors respond to decreased blood pressure by triggering increased sympathetic signaling and maintaining cardiac output (sympathetic “fight or flight” response). In cases of decompensated shock, when compensatory mechanisms falter and prior to the onset of death, the pumping action of the heart ceases, leading to a substantial decline in systemic arterial and venous pressures. Consequently, the arteriovenous pressure gradient diminishes [6,41,42]. This altered hemodynamic state results in stasis of CM in the venous system in the presence of the left chamber and arterial opacification, and of other infrequent and often unappreciated ominous MDCT vascular signs that represent a true hypovolemic state and must be recognized early by the radiological staff to improve survival [24,43,44,45,46,47,48]. This may be called the “venous CM pooling and layering” pattern, indicating that compensatory mechanisms are becoming insufficient and the patient must receive immediate treatment.
- (c)
-
In irreversible end-organ dysfunction, injected IV CM circulation is supported only by the pressure applied by the automated power injector and the density of contrast material. Circulatory arrest leads to dense contrast pooling and layering in the SVC, IVC (inferior vena cava), and right heart chambers with non-opacified left heart chambers or arterial vessels (Figure 1) [43,45,49,50,51,52]. This may be called the “non-beating heart” pattern. Cardio-pulmonary aggressive resuscitation must immediately be initiated within the framework of a predetermined emergency plan.
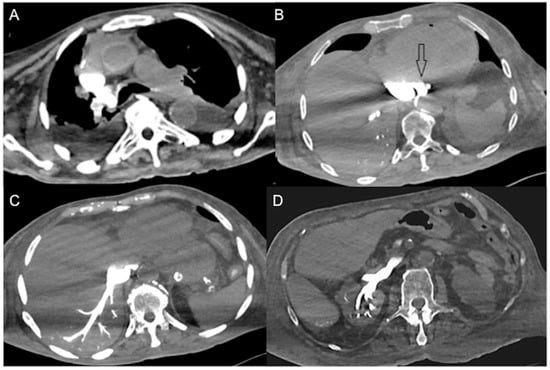 Figure 1. Non-beating heart in a 72-year-old man with sudden-onset severe dyspnea/shock and asystole during thoraco-abdominal CT. (A) CECT axial image shows dense contrast in the round superior vena cava, and reflux in the azygous arch; (B) contrast pooling and layering in the right atrium and IVC with retrograde opacification of coronary sinus (arrow). (C) CM fills the round inferior vena cava with hypostatic reflux into the hepatic veins, hemiazygos vein, partially splenic vein, and (D) right renal vein. Note no mixing of blood with CM and no opacification of the pulmonary arteries, aorta, and left cardiac chambers, suggestive of a non-beating heart. Prompt initiation of cardio-pulmonary resuscitation to restore circulation was useless. Autopsy: ruptured myocardial infarction.
Figure 1. Non-beating heart in a 72-year-old man with sudden-onset severe dyspnea/shock and asystole during thoraco-abdominal CT. (A) CECT axial image shows dense contrast in the round superior vena cava, and reflux in the azygous arch; (B) contrast pooling and layering in the right atrium and IVC with retrograde opacification of coronary sinus (arrow). (C) CM fills the round inferior vena cava with hypostatic reflux into the hepatic veins, hemiazygos vein, partially splenic vein, and (D) right renal vein. Note no mixing of blood with CM and no opacification of the pulmonary arteries, aorta, and left cardiac chambers, suggestive of a non-beating heart. Prompt initiation of cardio-pulmonary resuscitation to restore circulation was useless. Autopsy: ruptured myocardial infarction.
4. CT-Updated HSC Findings as Diagnostic Biomarkers
5. CECT Findings/Biomarkers as Prognostic Indicators
|
CECT Findings |
Cardiogenic |
Distributive |
Hypovolemic |
Obstructive |
Outcome |
|---|---|---|---|---|---|
|
Small-caliber aorta |
~25% |
~28% |
~30% |
~35% |
poor |
|
Slit/flattened cava |
~70% |
~55% |
~77% |
~50% |
very poor |
|
Halo sign IVC |
~70% |
~65% |
~75% |
NA |
poor |
|
Narrow SMA/V |
NA |
NA |
NA |
NA |
NA |
|
Lack of left AV enhancement |
~65% |
~35% |
~55% |
~20–50% |
very poor |
|
CM vascular layering |
~75% |
NA |
~65% |
~70% |
very poor |
|
Hot-spot sign |
NA |
NA |
NA |
NA |
NA |
|
Periportal halo |
~60% |
NA |
~40% |
NA |
NA |
|
Ongoing hemorrhage |
10% |
15% |
65% |
25% |
poor |
|
Shock Thyroid |
NA |
NA |
NA |
NA |
very poor |
|
Shock Lungs |
NA |
NA |
NA |
NA |
NA |
|
Shock Bowel |
~55% |
~50% |
~70% |
~40% |
poor |
|
Shock Spleen |
~40% |
~50% |
~50% |
~25% |
poor |
|
Liver altered density |
~85% |
~55% |
~57% |
~45% |
poor |
|
Shock gallbladder |
20–30% |
~12% |
13–35% |
~9% |
poor |
|
Shock pancreas |
~35% |
~55% |
~45% |
~35% |
very poor |
|
Shock Stomach |
NA |
NA |
NA |
NA |
NA |
|
Shock Kidneys |
~55% |
~50% |
~60% |
~40% |
poor |
|
Shock Adrenals |
~60% |
~65% |
~55% |
~50% |
poor |
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13132304
