Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Complementary therapies and phytonutrients derived from plant sources have been proposed as treatments for Parkinson’s disease. Numerous natural phytochemicals have emerged as therapeutically interesting compounds, drug entities, and phytochemicals for the treatment of inflammatory disorders. Additionally, numerous pharmacological studies have shown that phytochemicals are useful in treating neurodegenerative diseases (NDDs), depression, and dementia.
- neurodegeneration
- antioxidants
- phytoconstituents
1. Introduction
NDDs (neurodegenerative disorders) are a collection of disorders with various clinical implications and etiology. ALS (amyotrophic lateral sclerosis), cerebellar illness, Huntington’s chorea, Parkinson’s disease, Alzheimer’s, dementia, and schizophrenia are all examples of NDDs [1][2][3][4]. Age groups, hereditary diseases, non-enzymatic antioxidants, excitotoxicity, cytoskeletal abnormalities, autoimmunity, asymmetry, toxicity, elevated blood pressure, oxidative stress, and peripheral vascular diseases are subject to both experimental and epidemiological research. Some of the risk variables that have been discovered through the clinical manifestations of NDDs are associated with free radical toxicity, radical-mediated alteration, oxidase dysfunction, and endoplasmic reticulum stress caused by perinatal genetic abnormalities. The most important common symptoms are disturbances in balance, breathing, movement, reflexes, motor skills, or cardiac activity. Antioxidants such as flavonoids, polyphenols, and vitamins E and C can help to prevent these symptoms [5][6][7]. Antioxidants have a great impact on human health, as they can fight free radicals and, thus, halt the aging process and decrease the effects of oxidative damage caused by, for example, an unbalanced diet [8][9]. As a result, the long-term risk of neurodegenerative diseases is reduced (Figure 1). Although they can be treated, there is currently no cure for neurodegenerative diseases. Treatment of this disease relieves the symptoms in order to preserve the quality of life. Natural antioxidants, such as polyphenols, are becoming more popular because they can be obtained from foods and supplements and offer a range of health benefits [10]. Although it affects both males and females with increase in their age but males tend to be affected more frequently [11]. It is considered an international disease that does not distinguish between social class or race. The disease is estimated to affect 1% of people over the age of 65 worldwide, accounting for up to two-thirds of all people with movement disorders [12].
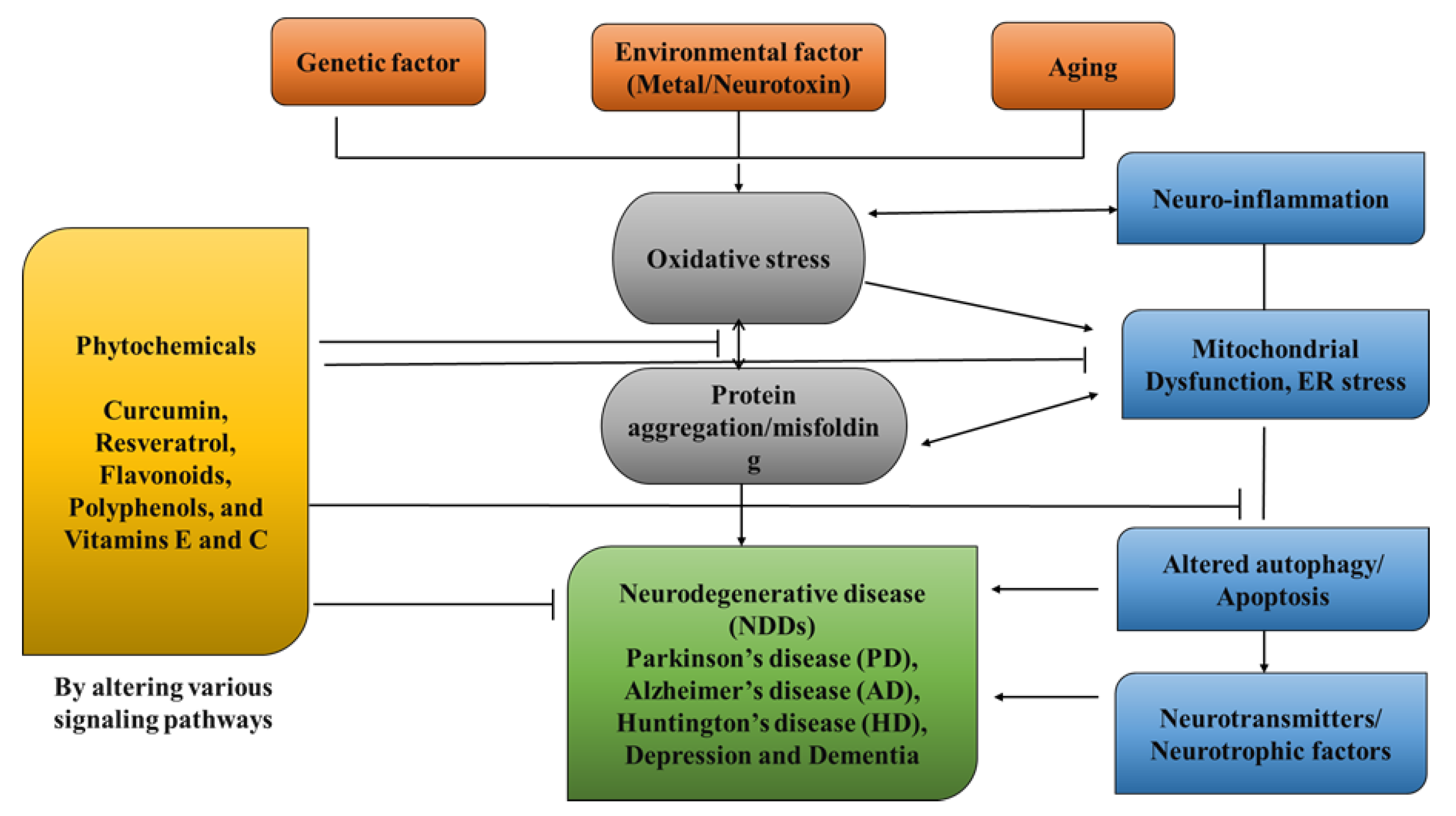
Figure 1. Role of Phytochemicals in the treatment of neurological diseases.
As the population ages, PD has become more prevalent, with those over 85 years old reaching 2.6%. The non-motor symptoms that patients with PD encounter include pain, exhaustion, autonomic dysfunction, changed mood, sleep disturbance, and cognitive abnormalities [13]. PD is referred to asa synucleinopathy because Lewy bodies, a crucial clinical characteristic of the disease, accumulate due to the misfolding of β-synuclein as a major feature. Furthermore, β-synuclein appears to be connected to both idiopathic and inherited forms of Parkinson’s disease and has a special role in the disease’s pathogenesis. It is worth noting that β-synuclein buildup has been closely linked to posttranslational modifications, systemic inflammation, oxidative stress, mitochondrial biogenesis, changed mitochondrial physical properties, synapse dysfunction, glycolipids, ER stress, and metal complexes. The overproduction of ROS and years of age breakdown the antioxidant defense system and increase oxidative stress in certainbrain areas, which can contribute to the misfolding of β-synuclein being the catalyst for the aging process in PD [14][15]. Although it is not a cure, levodopa has emerged as an efficacious drug for PD’s first motor symptoms. Despite the fact that stiffness and bradykinesia respond the best, tremors may only be somewhat reduced by levodopa. Other symptoms, such as balance issues, may worsen. L-dopa, however, cannot be used to treat Lewy disease, non-motor symptoms, or neuronal loss [16]. Patients require greater L-dopa doses over time, which is accompanied by a rise in side effects such as dyskinesias. Amantadine, an antiviral medication, appears to alleviate motor problems as well. Medicines based on dopamine agonists are also administered to address a range of neuromotor symptoms that are associated with disease progression. The long-term use of conventional PD medications can lead to adverse effects, such as dyskinesias and motor fluctuations (Table 1). As a result, novel therapeutic techniques used to prevent neurodegeneration, non-motor symptoms, Lewy disease accumulation, or synuclein aggregation in the brain are required [17][18].
Table 1. Side effects of synthetic drugs.
| Synthetic Drug | Toxicity | References |
|---|---|---|
| Levodopa | Motor complications, dyskinesias, motor fluctuations | [19] |
| Dopamine Agonists | Hallucinations, impulse control disorders, excessive daytime sleepiness | [20] |
| MAO-B Inhibitors | Potential hypertensive crises, interactions with medications and certain foods | [21] |
| Anticholinergic Drugs | Cognitive decline, memory impairment, dry mouth, urinary retention | [22] |
| COMT Inhibitors | Hepatotoxicity, liver dysfunction | [23] |
2. Complementary Therapies for PD
Complementary therapies and phytonutrients derived from plant sources have been proposed as treatments for Parkinson’s disease [24]. Numerous natural phytochemicals have emerged as therapeutically interesting compounds, drug entities, and phytochemicals for the treatment of inflammatory disorders [25]. Additionally, numerous pharmacological studies have shown that phytochemicals are useful in treating neurodegenerative diseases (NDDs), depression, and dementia [26][27][28][29][30]. Physiologically active phytochemicals are important therapeutically because they serve as the antioxidant defense system’s primary and secondary metabolites, which protect against a variety of stress-related disorders and clinical symptoms [31]. These phytochemicals’ positive and therapeutic effects include antioxidant capacity, pathogen prevention, immune system activation, and nutritional support for healthy living cells [32]. Botanical substances, with their active phytonutrients, efficiently salvage oxygen-based free radicals, influencing the antioxidative defense system and contributing to major cognitive impairments such as dyskinesias, residual tremors, muscle stiffness, and instability [33]. (Figure 2).
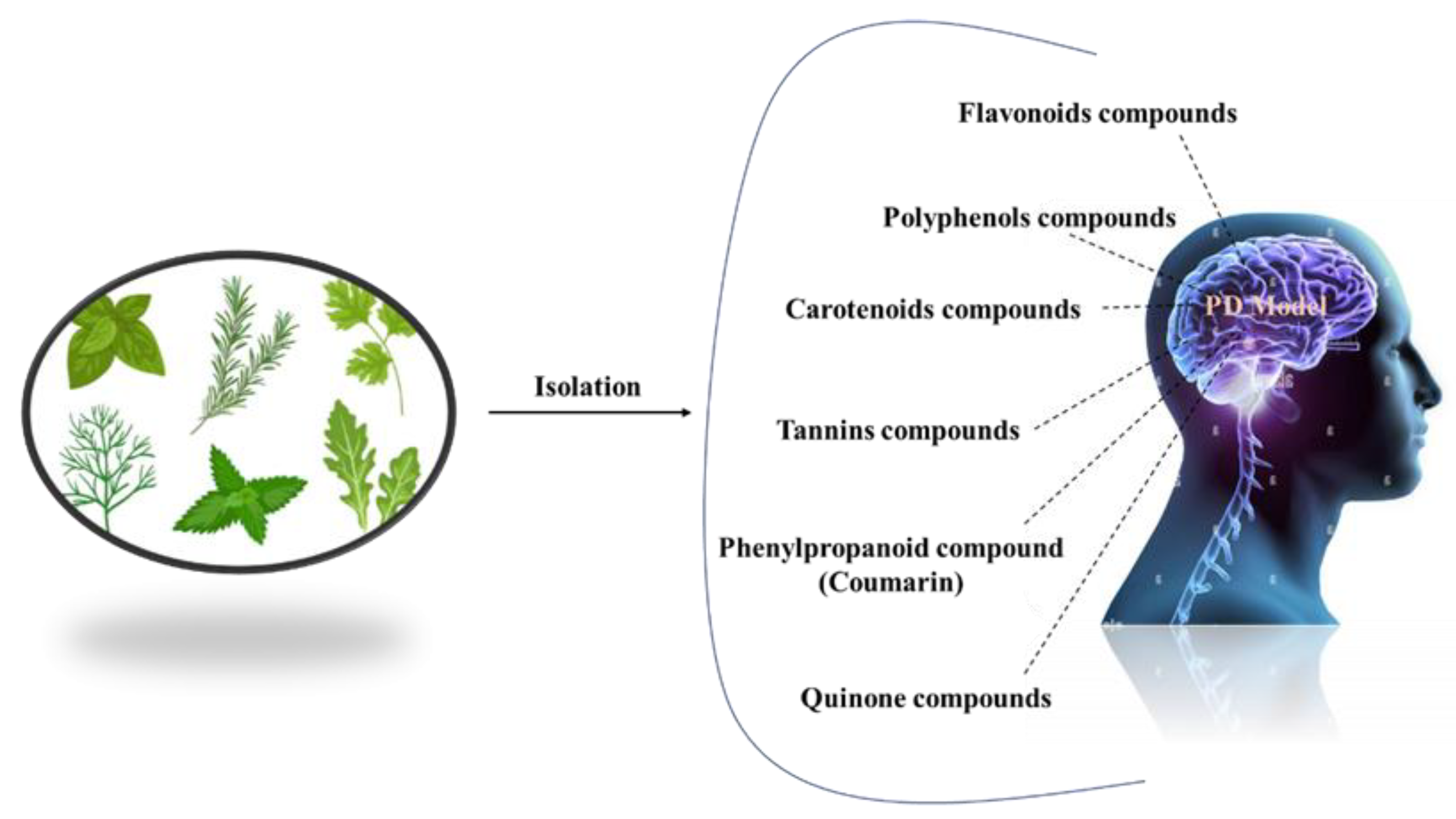
Figure 2. Different natural antioxidants used in the treatment of PD.
3. Pathogenesis
Dopamine levels in the caudal nucleus, striatum, and expression medium fall as a result of the death of dopaminergic neurons in the substantia nigra pars compacta, which is associated with Parkinson’s disease. The progressive loss of cholinergic neurons is the most significant pathogenic discovery in Parkinson’s disease sufferers’ brains. For this reason, with the loss of these neurons, the dopamine levels fall. When 50–60% of the dopamine pathway is shut down and striatal dopamine levels are lowered by 80–85%, symptoms of the disease arise. However, Parkinson’s disease may have multiple etiological factors, including mitochondrial dysfunction, neuroinflammation, oxidative stress, and the formation of protein kinases called Lewy bodies in the cytosol (Figure 3) [34].
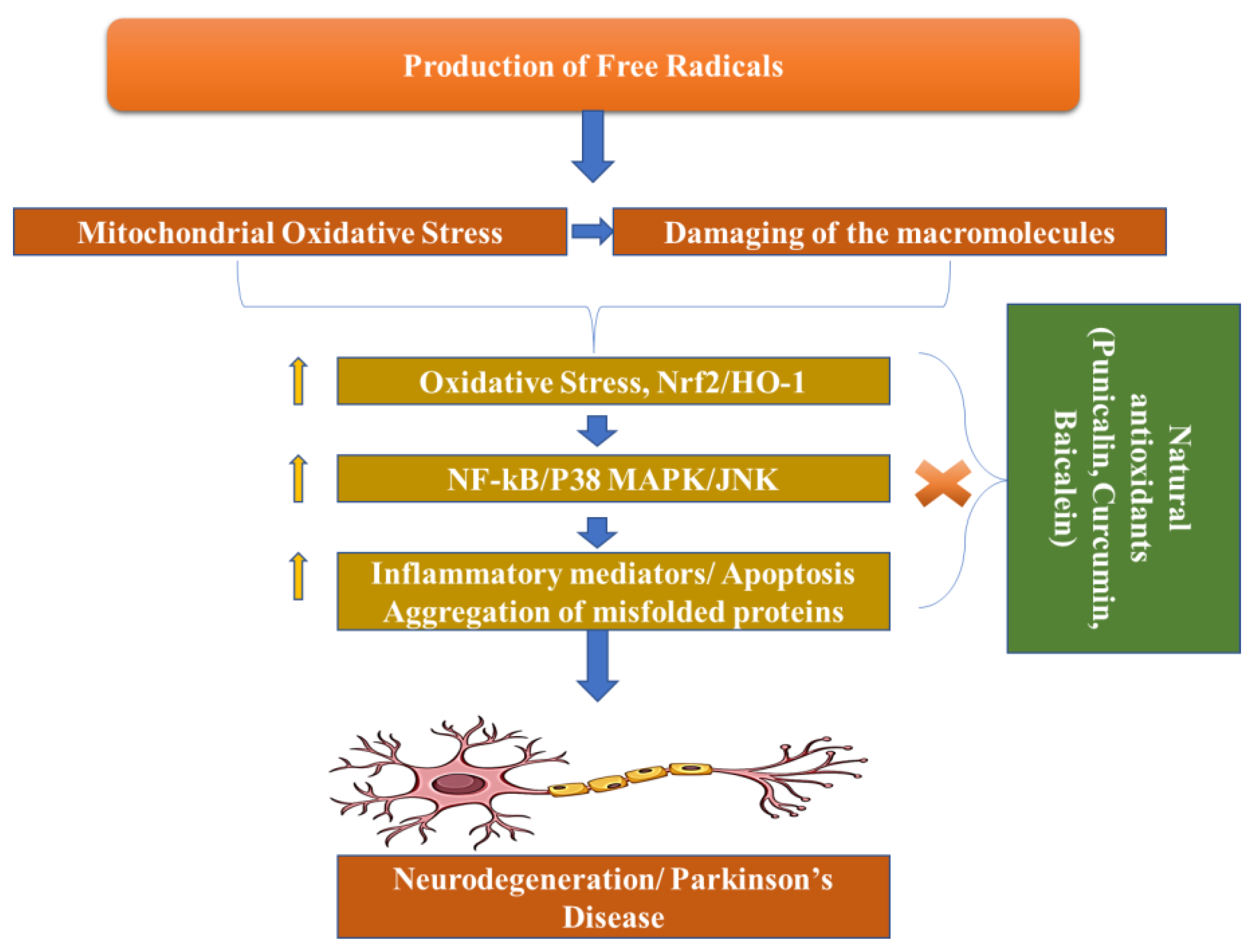
Figure 3. Pathogenesis of Parkinson’s Disease.
4. Phytochemicals and Parkinson’s Disease
Neurodegenerative diseases, including Parkinson’s disease, contributes to N increase in nicotinamide adenine dinucleotide phosphate oxidase and intracellular RNS, and, thus, result in cell damage due to oxidative stress [35].
Free radicals also damage cell membranes by oxidizing proteins, lipids, and nucleic acids, causing peptide cross-links [36]. The antioxidant and chelating effects of flavonoids and terpenoids are believed to be accountable for their therapeutic effects. Terpenoids, phenols, and flavonoids show important neuroprotective effects, due to their oxidative capacity [37]. By releasing extra protons, flavonoids scavenge superoxide, hydroxyl, and peroxyl radicals by donating an extra proton. This prevents ROS generation by the formation of complex structures containing dihydroxy groups, copper, multiple ions of transition metals, and iron [38].
Glutathione reductase, septo-optic dysplasia, and glutathione-S-transferase (GST) are the polyphenols that activate antioxidant enzymes. Polyphenols can raise the antioxidant levels by enhancing cell-signaling pathways through these enzymes. By generating unsaturated carbon–carbon double bonds with lipid bilayers, these phenolic acids inhibit lipid peroxidation and, thus, work as a powerful therapeutic and preventative agent [39].
The lack of oxygenation of the rings is assumed to be responsible for chrysin’s chemical capabilities, which include anti-inflammatory and antioxidant potential [40]. Diverse structures of flavones have been discovered to increase the capacity of antioxidant enzymes and also work in inhibiting COX-2, which is a pro-inflammatory mediator. Experiments with xanthine oxidase demonstrate that chrysin dramatically reduces ROS production. It is worth noting that rodent models require polyphenol dosages ranging from 10 µM to 100 µM to exercise their putative antioxidant and anti-inflammatory properties [41].
This entry is adapted from the peer-reviewed paper 10.3390/ph16070908
References
- Chauhan, S.; Gupta, S.; Yasmin, S.; Saini, M. Antihyperglycemic and Antioxidant Potential of Plant Extract of Litchi chinensis and Glycine max. Int. J. Nutr. Pharmacol. Neurol. Dis. 2021, 11, 225–233.
- Chauhan, S.; Kaur, N.; Kishore, L.; Singh, R. Pharmacological evaluation of anti-inflammatory and analgesic potential of Litchi chinensis gaertn.(sonn.). Group 2014, 10, 100.
- Karunaratne, T.B.; Okereke, C.; Seamon, M.; Purohit, S.; Wakade, C.; Sharma, A. Niacin and butyrate: Nutraceuticals targeting dysbiosis and intestinal permeability in parkinson’s disease. Nutrients 2020, 13, 28.
- Chattopadhyaya, I.; Gupta, S.; Mohammed, A.; Mushtaq, N.; Chauhan, S.; Ghosh, S. Neuroprotective effect of Spirulina fusiform and amantadine in the 6-OHDA induced Parkinsonism in rats. BMC Complement. Altern. Med. 2015, 15, 296.
- Sławińska, N.; Olas, B. Selected Seeds as Sources of Bioactive Compounds with Diverse Biological Activities. Nutrients 2023, 15, 187.
- Sharma, A.; Gupta, S.; Chauhan, S.; Nair, A.; Sharma, P. Astilbin: A promising unexplored compound with multidimensional medicinal and health benefits. Pharmacol. Res. 2020, 158, 104894.
- Sharma, C.; Chauhan, S.; Gupta, S.; Devi, A.; Nair, A. Role of whole plant extract of nelumbo nucifera gaertn in the treatment of thrombolysis. Cardiovasc. Hematol. Agents Med. Chem. 2019, 17, 115–124.
- Knight, E.; Geetha, T.; Burnett, D.; Babu, J.R. The Role of Diet and Dietary Patterns in Parkinson’s Disease. Nutrients 2022, 14, 4472.
- Sindhu, R.K.; Kaur, P.; Kaur, P.; Singh, H.; Batiha, G.E.-S.; Verma, I. Exploring multifunctional antioxidants as potential agents for management of neurological disorders. Environ. Sci. Pollut. Res. 2022, 29, 24458–24477.
- Evans, J.A.; Mendonca, P.; Soliman, K.F. Neuroprotective effects and therapeutic potential of the citrus flavonoid hesperetin in neurodegenerative diseases. Nutrients 2022, 14, 2228.
- Savica, R.; Grossardt, B.R.; Rocca, W.A.; Bower, J.H. Parkinson disease with and without dementia: A prevalence study and future projections. Mov. Disord. 2018, 33, 537–543.
- Choo, X.Y.; Lim, S.-Y.; Chinna, K.; Tan, Y.J.; Yong, V.W.; Lim, J.L.; Lau, K.F.; Chung, J.Y.; Em, J.M.; Tan, H.T. Understanding patients’ and caregivers’ perspectives and educational needs in Parkinson’s disease: A multi-ethnic Asian study. Neurol. Sci. 2020, 41, 2831–2842.
- Zeng, X.-S.; Geng, W.-S.; Jia, J.-J. Neurotoxin-induced animal models of Parkinson disease: Pathogenic mechanism and assessment. ASN Neuro 2018, 10, 1759091418777438.
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013, 14, 38–48.
- Kaur, I.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Aleya, L.; Bungau, S. Connecting the dots between mitochondrial dysfunction and Parkinson’s disorder: Focus mitochondria-targeting therapeutic paradigm in mitigating the disease severity. Environ. Sci. Pollut. Res. 2021, 28, 37060–37081.
- Chagraoui, A.; Boulain, M.; Juvin, L.; Anouar, Y.; Barrière, G.; De Deurwaerdère, P. L-DOPA in Parkinson’s disease: Looking at the “false” neurotransmitters and their meaning. Int. J. Mol. Sci. 2019, 21, 294.
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 17013.
- Ghosh, S.; Haldar, S.; Gupta, S.; Bisht, A.; Chauhan, S.; Kumar, V.; Roy, P.; Lahiri, D. Anisotropically conductive biodegradable scaffold with coaxially aligned carbon nanotubes for directional regeneration of peripheral nerves. ACS Appl. Bio Mater. 2020, 3, 5796–5812.
- Montastruc, J.; Rascol, O.; Senard, J.; Rascol, A. A randomised controlled study comparing bromocriptine to which levodopa was later added, with levodopa alone in previously untreated patients with Parkinson’s disease: A five year follow up. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1034–1038.
- Ahlskog, J.E.; Muenter, M.D.; Bailey, P.A.; Stevens, P.M. Dopamine agonist treatment of fluctuating parkinsonism: D-2 (controlled-release MK-458) vs combined D-1 and D-2 (pergolide). Arch. Neurol. 1992, 49, 560–568.
- Kumar, B.; Prakash Gupta, V.; Kumar, V. A perspective on monoamine oxidase enzyme as drug target: Challenges and opportunities. Curr. Drug Targets 2017, 18, 87–97.
- Mintzer, J.; Burns, A. Anticholinergic side-effects of drugs in elderly people. J. R. Soc. Med. 2000, 93, 457–462.
- Singer, C. Adverse effects in the treatment of Parkinson’s disease. Expert Rev. Neurother. 2002, 2, 105–118.
- Hussain, G.; Rasul, A.; Anwar, H.; Aziz, N.; Razzaq, A.; Wei, W.; Ali, M.; Li, J.; Li, X. Role of plant derived alkaloids and their mechanism in neurodegenerative disorders. Int. J. Biol. Sci. 2018, 14, 341.
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392.
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156.
- Mohseni, M.; Sahebkar, A.; Askari, G.; Johnston, T.P.; Alikiaii, B.; Bagherniya, M. The clinical use of curcumin on neurological disorders: An updated systematic review of clinical trials. Phytother. Res. 2021, 35, 6862–6882.
- Pearson, P.; Lewis, S.; Britton, J.; Fogarty, A. Vitamin E supplements in asthma: A parallel group randomised placebo controlled trial. Thorax 2004, 59, 652–656.
- Gao, W.; Tang, H.; Wang, D.; Zhou, X.; Song, Y.; Wang, Z. Effect of short-term vitamin D supplementation after nonsurgical periodontal treatment: A randomized, double-masked, placebo-controlled clinical trial. J. Periodontal Res. 2020, 55, 354–362.
- Vieregge, A.; Sieberer, M.; Jacobs, H.; Hagenah, J.; Vieregge, P. Transdermal nicotine in PD: A randomized, double-blind, placebo-controlled study. Neurology 2001, 57, 1032–1035.
- Chmiel, M.; Stompor-Gorący, M. The Spectrum of Pharmacological Actions of Syringetin and Its Natural Derivatives—A Summary Review. Nutrients 2022, 14, 5157.
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39.
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection against oxidative stress: Phytochemicals targeting TrkB signaling and the Nrf2-ARE antioxidant system. Front. Mol. Neurosci. 2020, 13, 116.
- Lees, A.J. Impact Commentaries. A modern perspective on the top 100 cited JNNP papers of all time: The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease: Accuracy of clinical diagnosis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2012, 83, 954–955.
- Mischley, L.K.; Standish, L.J.; Weiss, N.S.; Padowski, J.M.; Kavanagh, T.J.; White, C.C.; Rosenfeld, M.E. Glutathione as a biomarker in Parkinson’s disease: Associations with aging and disease severity. Oxidative Med. Cell. Longev. 2016, 2016, 9409363.
- Pospíšil, P.; Prasad, A.; Rác, M. Mechanism of the formation of electronically excited species by oxidative metabolic processes: Role of reactive oxygen species. Biomolecules 2019, 9, 258.
- Yi, L.; Ma, S.; Ren, D. Phytochemistry and bioactivity of Citrus flavonoids: A focus on antioxidant, anti-inflammatory, anticancer and cardiovascular protection activities. Phytochem. Rev. 2017, 16, 479–511.
- Chu, K.O.; Chan, S.-O.; Pang, C.P.; Wang, C.C. Pro-oxidative and antioxidative controls and signaling modification of polyphenolic phytochemicals: Contribution to health promotion and disease prevention? J. Agric. Food Chem. 2014, 62, 4026–4038.
- Zhang, Y.; Yu, W.; Zhang, L.; Wang, M.; Chang, W. The Interaction of Polyphenols and the Gut Microbiota in Neurodegenerative Diseases. Nutrients 2022, 14, 5373.
- Fadaka, A.O.; Ajiboye, B.O.; Adewale, I.; Ojo, O.A.; Oyinloye, B.E.; Okesola, M.A. Significance of antioxidants in the treatment and prevention of neurodegenerative diseases. J. Phytopharm. 2019, 8, 75–83.
- Muszyńska, B.; Łojewski, M.; Rojowski, J.; Opoka, W.; Sułkowska-Ziaja, K. Natural products of relevance in the prevention and supportive treatment of depression. Psychiatr. Pol. 2015, 49, 435–453.
This entry is offline, you can click here to edit this entry!
