Most abdominal masses in the pediatric population derive from the ovaries. Ovarian masses, which include both non-neoplastic lesions and neoplastic tumors, can occur in all ages although their incidence, clinical presentation and histological distribution vary among different age groups. Herein, pediatric non-neoplastic ovarian masses are described. These include benign tumor-like lesions that are not composed of neoplastic cells, such as functional cysts, endometrioma, torsion, abscess and lymphangioma.
- ovarian masses
- children
- adolescents
- imaging
1. Introduction
Abdominal masses in the pediatric population most commonly derive from the ovaries [1]. Ovarian masses, including both non-neoplastic lesions and neoplastic tumors, can occur in all age groups. The incidence, clinical presentation and histological distribution of such lesions in children and adolescents are distinct from those in adults and require a particularized therapeutic approach [2]. Masses of the ovary range from simple functional cysts to malignant neoplasms. Underneath, pediatric non-neoplastic ovarian masses are presented. These represent benign tumor-like lesions that are not composed of neoplastic cells and include functional cysts, endometrioma, torsion, abscess and lymphangioma.
2. Functional Cysts
3. Endometrioma
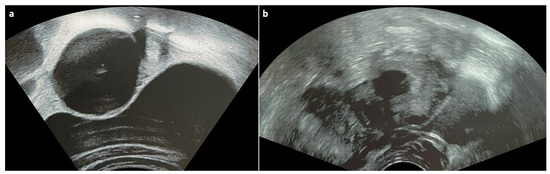
Endometriomas are cystic lesions that arise from the disease process of endometriosis, which is defined as the presence of endometrial glands and stroma outside the uterus [6]. They contain a thick, dark brown, endometrial fluid that results from the accumulation of degenerated menstrual blood products and are, therefore, often referred to as chocolate cysts [7]. Endometriomas most commonly develop in the ovaries and can affect almost all ages, although they are typically diagnosed during the reproductive years [8][9]. Clinical manifestations include pelvic pain or tenderness, dysmenorrhea, dyspareunia, painful urination, painful defecation, urinary frequency, nausea/vomiting, back pain, palpable mass during bimanual examination in large enough lesions and acute abdomen in case of rupture [9]. Patients with any of these findings usually undergo an ultrasound examination, which, in case of ovarian endometrioma, classically reveals a simple, unilocular, avascular cyst with ground-glass appearance, namely low-level homogenous echoes that result from old hemorrhagic debris (Figure 2) [10]. On magnetic resonance imaging (MRI), endometriomas typically appear T1 hyperintense without loss of signal after fat saturation and T2 hypointense. The low signal intensity on T2-weighted images, which is caused by the high concentration of protein and iron that result from cyclical hemorrhage, is referred to as the “shading sign” and, combined with high T1 signal intensity, is highly suggestive of endometrioma [11]. Treatment options for ovarian endometrioma include hormonal medications and surgery [9].
Figure 2. Ultrasonogram of a 17-year-old patient showing a typical unilocular endometrioma with ground-glass echogenicity of the cyst fluid.
4. Ovarian Torsion
5. Tubo-Ovarian Abscess
Tubo-ovarian abscess (TOA) is a complex, infectious, adnexal mass that results from PID and consequently affects sexually active patients usually aged 15–25 years [20]. It can manifest with abdominal pain, pelvic palpable mass, fever, nausea/vomiting, vaginal discharge or abnormal bleeding and cervical motion tenderness during bimanual examination. Further evaluation may show leukocytosis and bacterial growth in cervical, urine and blood cultures [21]. On imaging, TOA appears as a complex fluid-filled lesion with thick walls and septa [14]. US reveals an adnexal mass with mixed or ground-glass echogenicity [5]. On MRI, the fluid of TOA exhibits variable signal intensity on T1-weighted images, reflecting the degree of proteinaceous and hemorrhagic contents and often appears hyperintense on T2-weighted images. The walls and septa typically show low T1 and T2 signal intensity and enhance avidly [14]. T1-weighted imaging may demonstrate an internal hyperintense and enhancing rim, which is supposed to represent granulation tissue combined with hemorrhage [22]. Restricted diffusion is observed on diffusion-weighted imaging (DWI) [14]. Since TOA occurs in the context of PID, imaging can also depict adnexal edema and peritoneal fibrosis and adhesions, which appear as mesh-like linear strands with low T1 signal intensity and enhancement [22]. The combination of the aforementioned clinical and radiological characteristics render the diagnosis of TOA relatively straightforward. TOA is typically treated with antibiotics, although surgery may be unavoidable in some cases [21].
6. Lymphangioma
Lymphangiomas are rare benign malformations of the lymphatic system [23]. Their localization in the ovary is uncommon [24]. In fact, only 24 cases of ovarian lymphangioma have been reported in the literature during the last 70 years, the majority of which concerned adult patients [24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47]. Pani et al. [24] reported a case of a 16-year-old girl that presented with a longstanding, painless, abdominal distension and a non-tender, mobile, palpable mass of the entire abdomen. Tumor markers were negative. CT and MRI depicted a large, multiseptated, fluid-filled, cystic mass with well-defined and non-enhanced walls that measured 40 cm in the longitudinal axis and did not infiltrate into the surrounding tissues. The lesion was surgically removed, and the final diagnosis after histopathological examination was lymphangioma arising from the left ovary. The postoperative course of the patient was uneventful. This case highlights that lymphangiomas can occur in the pediatric population and should be included in the differential diagnosis of ovarian masses in this age group.
This entry is adapted from the peer-reviewed paper 10.3390/children10071114
References
- Ciro, E.; Vincenzo, C.; Mariapina, C.; Fulvia, D.C.; Vincenzo, B.; Giorgia, E.; Roberto, C.; Lepore, B.; Castagnetti, M.; Califano, G.; et al. Review of a 25-Year Experience in the Management of Ovarian Masses in Neonates, Children and Adolescents: From Laparoscopy to Robotics and Indocyanine Green Fluorescence Technology. Children 2022, 9, 1219.
- Tarca, E.; Trandafir, L.M.; Cojocaru, E.; Costea, C.F.; Rosu, S.T.; Butnariu, L.I.; Iordache, A.C.; Munteanu, V.; Luca, A.C. Diagnosis Difficulties and Minimally Invasive Treatment for Ovarian Masses in Adolescents. Int. J. Womens Health 2022, 14, 1047–1057.
- Mobeen, S.; Apostol, R. Ovarian Cyst. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- User, İ.R.; Karakuş, S.C.; Özokutan, B.H.; Akçaer, V.; Burulday, B.; Ceylana, H. Can Preoperative Findings Help to Interpret Neoplastic and Non-Neoplastic Lesions of Ovary and Affect Surgical Decisions in Children and Adolescents? Arch Argent Pediatr. 2019, 117, 294–400.
- Sayasneh, A.; Ekechi, C.; Ferrara, L.; Kaijser, J.; Stalder, C.; Sur, S.; Timmerman, D.; Bourne, T. The Characteristic Ultrasound Features of Specific Types of Ovarian Pathology (Review). Int. J. Oncol. 2015, 46, 445–458.
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obs. Gynecol. Rep. 2017, 6, 34–41.
- Guo, S.-W.; Ding, D.; Shen, M.; Liu, X. Dating Endometriotic Ovarian Cysts Based on the Content of Cyst Fluid and Its Potential Clinical Implications. Reprod. Sci. 2015, 22, 873–883.
- Bortoletto, P.; Pollie, M. Management of Ovarian Endometrioma in Asymptomatic Reproductive Age Women. Curr. Obstet. Gynecol. Rep. 2021, 10, 53–60.
- Hoyle, A.T.; Puckett, Y. Endometrioma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Van Holsbeke, C.; Van Calster, B.; Guerriero, S.; Savelli, L.; Paladini, D.; Lissoni, A.A.; Czekierdowski, A.; Fischerova, D.; Zhang, J.; Mestdagh, G.; et al. Endometriomas: Their Ultrasound Characteristics. Ultrasound Obs. Gynecol. 2010, 35, 730–740.
- Thalluri, A.L.; Knox, S.; Nguyen, T. MRI Findings in Deep Infiltrating Endometriosis: A Pictorial Essay. J. Med. Imaging Radiat. Oncol 2017, 61, 767–773.
- Sintim-Damoa, A.; Majmudar, A.S.; Cohen, H.L.; Parvey, L.S. Pediatric Ovarian Torsion: Spectrum of Imaging Findings. RadioGraphics 2017, 37, 1892–1908.
- Chang, H.C.; Bhatt, S.; Dogra, V.S. Pearls and Pitfalls in Diagnosis of Ovarian Torsion. RadioGraphics 2008, 28, 1355–1368.
- Lam, C.Z.; Chavhan, G.B. Magnetic Resonance Imaging of Pediatric Adnexal Masses and Mimics. Pediatr. Radiol. 2018, 48, 1291–1306.
- Ngo, A.-V.; Otjen, J.P.; Parisi, M.T.; Ferguson, M.R.; Otto, R.K.; Stanescu, A.L. Pediatric Ovarian Torsion: A Pictorial Review. Pediatr. Radiol. 2015, 45, 1845–1855.
- Gross, M.; Blumstein, S.L.; Chow, L.C. Isolated Fallopian Tube Torsion: A Rare Twist on a Common Theme. Am. J. Roentgenol. 2005, 185, 1590–1592.
- Oltmann, S.C.; Fischer, A.; Barber, R.; Huang, R.; Hicks, B.; Garcia, N. Cannot Exclude Torsion—A 15-Year Review. J. Pediatr. Surg. 2009, 44, 1212–1217.
- Cass, D.L. Ovarian Torsion. Semin. Pediatr. Surg. 2005, 14, 86–92.
- Servaes, S.; Zurakowski, D.; Laufer, M.R.; Feins, N.; Chow, J.S. Sonographic Findings of Ovarian Torsion in Children. Pediatr. Radiol. 2007, 37, 446–451.
- Chen, K.-Y.; Tseng, J.-Y.; Yang, C.-Y. Tubo-Ovarian Abscess with Sepsis in a Nonagenarian Woman: A Case Report and Literature Review. BMC Women’s Health 2019, 19, 81.
- Kairys, N.; Roepke, C. Tubo-Ovarian Abscess. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Ha, H.K.; Lim, G.Y.; Cha, E.S.; Lee, H.G.; Ro, H.J.; Kim, H.S.; Kim, H.H.; Joo, S.W.; Jee, M.K. MR Imaging of Tubo-Ovarian Abscess. Acta Radiol. 1995, 36, 510–514.
- Miceli, A.; Stewart, K.M. Lymphangioma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Pani, E.; Martin, A.; Buccoliero, A.; Ghionzoli, M.; Messineo, A. Giant Ovarian Lymphangioma: Case Report and Review of the Literature. Fetal Pediatr. Pathol. 2018, 37, 263–269.
- Akyildiz, E.U.; Peker, D.; Ilvan, S.; Calay, Z.; Cetinaslan, I.; Oruc, N. Lymphangioma of the Ovary: A Case Report and Review of the Literature. J. BUON 2006, 11, 91–93.
- Iwasa, T.; Tani, A.; Miyatani, Y.; Bekku, S.; Yamashita, M.; Nakanishi, K.; Fujii, Y.; Ino, H. Lymphangioma of the Ovary Accompanied by Chylous Ascites. J. Obstet. Gynaecol. Res. 2009, 35, 812–815.
- Singer, T.; Filmar, G.; Jormark, S.; Seckin, T.; Divon, M. Rare Case of Ovarian Cystic Lymphangioma. J. Minim. Invasive Gynecol. 2010, 17, 97–99.
- Ferrari, W.; De Angelis, V. Lymphangioma of the ovary. Rev. Bras. Cir. 1953, 25, 329–334.
- Bieniasz, A.; Sierant, E. A Case of Lymphangioma Cavernosum of the Ovary. Ginekol. Pol. 1961, 32, 667–669.
- Palliez, R.; Delecour, M.; Dupont, A.; Monnier, J.; Begueri, F.; Houcke, M. Ovarian Lymphangioma. A Case. Bull. Fed. Des Soc. Gynecol. Dobstetrique Lang. Fr. 1970, 22, 51–53.
- Aristizabal, S.A.; Galindo, J.H.; Davis, J.R.; Boone, M.L. Lymphangiomas Involving the Ovary. Report of a Case and Review of the Literature. Lymphology 1977, 10, 219–223.
- Khanna, S.; Mehrotra, M.L.; Basumallick, M.K. Lymphangioma Cavernosum of the Ovary. Int. Surg. 1978, 63, 104–105.
- Logani, K.B.; Agarwal, K. Lymphangioma of the Ovary. J. Indian Med. Assoc. 1997, 95, 146–152.
- Evans, A.; Lytwyn, A.; Urbach, G.; Chapman, W. Bilateral Lymphangiomas of the Ovary: An Immunohistochemical Characterization and Review of The Literature. Int. J. Gynecol. Pathol. 1999, 18, 87–90.
- Ahluwalia, J.; Girish, V.; Saha, S.; Dey, P. Lymphangioma of the Ovary. Acta Obs. Gynecol. Scand. 2000, 79, 894–895.
- Kearney, C.E.; Hall, G.H.; Purdie, D.W.; Turnbull, L.W. Ovarian Lymphangioma: MRI Appearances. Clin. Radiol. 2001, 56, 685–687.
- Heinig, J.; Beckmann, V.; Bialas, T.; Diallo, R. Lymphangioma of the Ovary after Radiation Due to Wilms’ Tumor in the Childhood. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 103, 191–194.
- Park, C.; Lee, J.W.; Kim, S.J.; Kim, J. Sonographic Findings of Prenatal Torsion of Ovarian Lymphangioma. J. Clin. Ultrasound 2005, 33, 421–423.
- Jain, D.; Saroha, V.; Singh, M. Lymphangioma of the Ovary. J. Obstet. Gynaecol. 2009, 29, 260–261.
- Jallouli, M.; Trigui, L.; Gouiaa, N.; Gargouri, A.; Mhiri, R. Neonatal Ovarian Lymphangioma. J. Pediatr. Adolesc. Gynecol. 2011, 24, e9–e10.
- Naik, S. Rare Case of Ovarian Cystic Lymphangioma Managed at Laparoscopy. J Gynec Endosc Surg 2011, 2, 97.
- Pillai, S.; O’Brien, D.; Stewart, C.J.R. Bilateral Ovarian Lymphangioma (Lymphangioleiomyoma). Int. J. Gynecol. Pathol. 2013, 32, 171–175.
- Goyal, S.; Sharma, S.; Kotru, M.; Sharma, A. Ovarian Lymphangioma Masquerading as Ectopic Pregnancy: A Clinical Dilemma. J. Obstet. Gynaecol. 2015, 35, 535–536.
- Sinhasan, S.; Nagesha, K. Intra-Abdominal Cystic Lymphangioma in an Adult Female Masquerading Ovarian Tumor. Indian J. Cancer 2015, 52, 380.
- Radhouane, A.; Mayada, S.; Khaled, N. Lymphangioma of the Ovary: Etiology and Management. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 203, 342–343.
- Choudhary, R.A.; Vora, P.H.; Deodhar, K.K.; Pisat, S.V.; Ganla, M.K.; Ganla, K.N. Rare Case of Bilateral Ovarian Lymphangioma with Chylous Ascites in Pregnancy with Review of Literature. J. Obs. Gynecol. India 2021, 71, 184–187.
- Vaidya, K.M.; Shrestha, B. Ovarian Lymphangioma with Mature Cystic Teratoma. J. Nepal. Health Res. Counc. 2019, 17, 128–130.
