Zeolites are crystalline, hydrated aluminosilicates with an open-framework structure. Unique structural features make them very useful ion-changers, adsorbents and catalysts. The catalytic use of zeolites has expanded from traditional use in the petrochemical industry and refineries to use in the catalytic degradation of various environmental pollutants and the synthesis of fine chemicals. Progress on the use of zeolites has been achieved in biomass conversion to fuels and valuable industrial bio-based chemicals.
- clinoptilolite
- natural zeolite
- catalysis
1. Introduction
Today, environmental pollution is one of the most important topics of worldwide discussion. An increase in the population along with rapid industrialization and urbanization have led to damage of the environment and consequently to serious damage to human health. Furthermore, the situation is becoming more complex due to insufficient attention and control of the discharging of many pollutants to the environment, such as heavy metals, pharmaceuticals, pesticides, organic dyes, etc. Many of these pollutants are persistent, toxic and carcinogenic. Also, the generation of large amounts of solid waste and its inappropriate disposal negatively affect the environment.
Consequently, research efforts are focused on developing new materials and technologies that can minimize environmental pollution. According to the principles of sustainable development, they are necessary to be not only effective but also environmentally and economically acceptable. In this regard, catalysis is one of the main fields that can give a significant contribution to the field of green chemistry and environmental protection. About 90% of all industrial processes are catalyzed, so the choice of catalyst is one of the key parameters for the sustainability of applied technologies. A large number of both homogeneous and heterogeneous catalysts are in use. Different kinds of solids, including activated carbon-based materials, mesoporous silica, clays, zeolites and zeolite-like materials have been studied in catalysis [1][2][3][4][5][6][7].
Zeolites are hydrated aluminosilicates with unique structural features and a chemical composition that is applicable in many areas. In the second part of the 20th century, there was an expansion of synthetic zeolites with new structural features, which led to the neglect of natural zeolites in many scientific studies. However, the 21st century, as the century of green chemistry, brought natural zeolites back into the spotlight of scientific interest. Many regions in the world have deposits with high contents of zeolites with high purity. Due to their low price and availability, they have become a good basis for the development of new green adsorbents and catalysts.
Zeolites are hydrated aluminosilicates with unique structural features and a chemical composition that is applicable in many areas. In the second part of the 20th century, there was an expansion of synthetic zeolites with new structural features, which led to the neglect of natural zeolites in many scientific studies. However, the 21st century, as the century of green chemistry, brought natural zeolites back into the spotlight of scientific interest. Many regions in the world have deposits with high contents of zeolites with high purity. Due to their low price and availability, they have become a good basis for the development of new green adsorbents and catalysts.
2. A Brief Description of Zeolite Structures
Zeolites are crystalline, open-framework aluminosilicates. They are composed of three-dimensional frameworks consisting of [SiO4]4– and [AlO4]5– tetrahedral units linked via oxygen atoms (Figure 1). The lattice of zeolites is negatively charged, and its electroneutrality is achieved by extraframework cations - alkali and earth alkaline cations. These cations interact with the lattice via electrostatic interactions and are movable, which gives zeolites an ion-exchange property. Ion-exchange takes place to varying degrees depending on nature of zeolites, nature of cations present in zeolite lattice and solution as well as on experimental conditions such as static or dynamic regime, solid:liquid ratio, contact time, reaction temperature, initial concentration, pH, contact time.
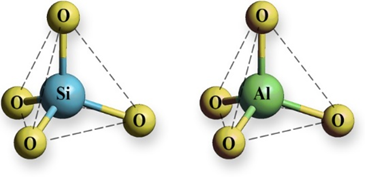
Figure 1. [SiO4]4− and [AlO4]5− tetrahedral units of a zeolite lattice.
Porosity of the lattice provides a large specific area, being several hundred to thousand square meters per gram, which gives zeolites good adsorptive properties. The shape and openings of cavities and channels affect the adsorptive behavior allowing only species of proper dimensions and geometries to enter and diffuse throughout the lattice.
Zeolites show also catalytic properties. Hydrogen ions can be accommodated in the zeolite lattice instead of metal cations. This brings Brönsted acidity and makes zeolites catalytically active. The presence of three-coordinated Al and/or extra-framework Al species in the lattice brings Lewis acid sites (Figure 2). The acidity of zeolites can be significantly enriched through different chemical and/or thermal treatments.
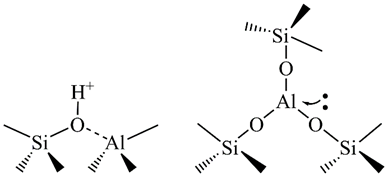
Figure 2. The Brönsted (left) and Lewis (right) acid sites in a zeolite lattice.
3. Clinoptilolite
According to the structural database of the International Zeolite Association (IZA), currently about 60 different zeolite structures belong to natural zeolites and 248 for synthetic ones[8]. Among the natural zeolites, clinoptilolite (CLI) is one of the most abundant and the most widely studied natural zeolites. Deposits of CLI-rich tuffs are found in many countries including deposits in China, the United States, Indonesia, New Zealand, Cuba and the Republic of Korea. In Europe, the abundant deposits are found in Turkey, Hungary, Slovenia, Slovakia, Ukraine, Italy, Romania and Serbia[9][10][11]. Depending on the location of deposits, the CLI content varies between 60% and 90%, whereas feldspars, clays and quartz are the most common present satellite phases.
CLI is a member of the heulandite (HEU) group of natural zeolites. CLI and HEU are isostructural and differ only by the Si/Al molar ratio, which influences their thermal stability. The Si/Al ratio of CLI is in the range 4.0–5.3, in contrast to HEU, for which this ratio is lower than 4.0[9][12][13]. A higher Si/Al ratio of CLI makes CLI more thermally stable (up to 800 °C) than HEU (up to 550 °C).
The framework of the CLI consists of three types of channels: A - formed from 10-membered rings, B - formed from 8-membered rings and C - formed from 8-membered rings. The channels A (0.3 x 0.76 nm) and B (0.33 x 0.46 nm) are parallel to the c-axis, while the C channel (0.26 x 0.47 nm) is parallel to the a-axis[14][15].
3.1. Modification of CLI
Due to its structural features and availability, CLI has been recognized as a perspective candidate for the synthesis of different types of materials that have been used in ion-exchange, adsorption and catalysis. In order to expand the range of CLI applications its modification by various chemical and/or thermal treatments has received much attention in many research works.
This entry is adapted from the peer-reviewed paper 10.3390/min13070877
References
- Perez, F.M.; Santori, G.F.; Pompeo, F.; Nichio, N.N. Silica-resin-bentonite nanocomposite and its application in catalysis. Minerals 2022, 12(12), 1486. https://doi.org/10.3390/min12121486
- Valášková, M.; Kočí, K.; Madejová, J.; Matějová, L.; Pavlovský, J.; Barrocas, B.T.; Klemencová, K. α-Fe2O3 nanoparticles/iron-containing vermiculite composites: Structural, textural, optical and photocatalytic properties. Minerals 2022, 12, 607. https://doi.org/10.3390/min12050607
- Li, X.; Peng, K. MoSe2/Montmorillonite composite nanosheets: Hydrothermal synthesis, structural characteristics, and enhanced photocatalytic activity. Minerals 2018, 8(7), 268. https://doi.org/10.3390/min8070268
- Di Lorenzo, F.; Ruiz-Aguido, C.; Ibañez-Velasco, A.; Gil-San Millan, R.; Navarro, J.A.R.; Ruiz-Agudo, E.; Rodriguez-Navarro, C. The carbonation of wollastonite: A model reaction to test natural and biomimetic catalysts for enhanced CO2 sequestration. Minerals 2018, 8(5), 209. https://doi.org/10.3390/min8050209
- Yu, X.; Williams, C.T. Recent advances in the applications of mesoporous silica in heterogeneous catalysis. Catal. Sci. Technol. 2022, 12, 5765–5794. https://doi.org/10.1039/D2CY0000Li, Y.; Yu, J. Emerging applications of zeolites in catalysis, separation and host–guest assembly. Nat. Rev. Mater. 2021, 6, 1156–1174. https://doi.org/10.1038/s41578-021-00347-3Baloyi, J.; Ntho, T.; Moma, J. Synthesis and application of pillared clay heterogeneous catalysts for wastewater treatment: a review. RSC Adv. 2018, 8, 5197–5211. https://doi.org/10.1039/C7RA12924F
- Li, Y.; Yu, J. Emerging applications of zeolites in catalysis, separation and host–guest assembly. Nat. Rev. Mater. 2021, 6, 1156–1174. https://doi.org/10.1038/s41578-021-00347-3Baloyi, J.; Ntho, T.; Moma, J. Synthesis and application of pillared clay heterogeneous catalysts for wastewater treatment: a review. RSC Adv. 2018, 8, 5197–5211. https://doi.org/10.1039/C7RA12924F
- Baloyi, J.; Ntho, T.; Moma, J. Synthesis and application of pillared clay heterogeneous catalysts for wastewater treatment: a review. RSC Adv. 2018, 8, 5197–5211. https://doi.org/10.1039/C7RA12924F
- https://europe.iza-structure.org/IZA-SC/ftc_table.php (accessed on 13 May 2023)
- Ambrozova, P.; Kynicky, J.; Urubek, T., Nguyen, V.D. Synthesis and modifications of clinoptilolite. Molecules 2017, 22(7), 1107. https://doi.org/10.3390/molecules22071107
- Mormone, A.; Piochi, M. Mineralogy, geochemistry and genesis of zeolites in cenozoic pyroclastic flows from the Asuni Area (Central Sardinia, Italy). Minerals 2020, 10(3), 268. https://doi.org/10.3390/min10030268
- Kowalczyk P.; Sprynskyy, M.; Terzyk, A.P.; Lebedynets, M.; Namieśnik, J.; Buszewski, B. Porous structure of natural and modified clinoptilolites. J. Colloid. Interface Sci. 2006, 297(1), 77–85. https://doi.org/10.1016/j.jcis.2005.10.045
- Mansouri, N.; Rikhtegar, N.; Panahi, S.H.; Atabi, F.; Shahraki, B.K. Porosity, characterization and structural properties of natural zeolite—Clinoptilolite—As a sorbent. Environ. Prot. Eng. 2013, 39, 139–152. DOI: 10.5277/EPE13011
- Godelitsas, A.; Armbruster, T. HEU-type zeolites modified by transition elements and lead. Micropor. Mesopor. Mat. 2003, 61, 3–24. https://doi.org/10.1016/S1387-1811(03)00352-4
- Dziedzicka, A.; Sulikowski, B.; Ruggiero-Mikolajczyk, M. Catalytic and physicochemical properties of modified natural clinoptilolite, Catal. Today 2016, 259, 50–58. https://doi.org/10.1016/j.cattod.2015.04.039
- Rajic, N.; Stojakovic, Dj.; Daneu, N.; Recnik, A. The formation of oxide nanoparticles on the surface of natural clinoptilolite, J. Phys. Chem. Solids 2011, 72, 800–803. https://doi.org/10.1016/j.jpcs.2011.03.018
