Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Matrix metalloproteinases (MMPs) are a large family of Ca2+ and Zn2+ dependent proteolytic enzymes, able to cleave the various components of the extracellular matrix (ECM), as well as a range of other regulatory molecules. It has proven the important role of both MMPs and their endogenous inhibitors, tissue inhibitors of metalloproteinases (TIMPs), in oral health, the initial development of the tooth, and during enamel maturation.
- Matrix metalloproteinases
- tissue inhibitors of metalloproteinases
- Periodontal Diseases
1. Introduction
Matrix metalloproteinases (MMPs) are a type of Ca2+- and Zn2+-dependent proteolytic enzymes. They are also called “matrixins”, and their main role is to cleave the various components of the extracellular matrix (ECM), as well as a range of other molecules, such as growth factors, cytokines, chemokines, and adhesion proteins [1]. In humans, the MMP family consists of more than 23 members, divided into six groups: collagenases, gelatinases, matrilysins, stromelysins, membrane-type MMPs, and other MMPs [2].
A variety of cell types are able to express and secrete MMPs as the main producers are the activated neutrophils, macrophages, endothelial cells, epithelial cells, vascular smooth muscle cells, glial cells, tumor cells, etc. [1].
Usually, after the activation of the cells, MMPs are released together with regulatory molecules such as interleukins (IL-8 and IL-1β), tumor necrosis factor (TNF)-α, osteoprotegerin (OPG), prostaglandins (PGE2), and receptor activator of nuclear factor kappa-B ligand (RANKL) [3].
The MMPs are involved in a vast range of physiological processes: angiogenesis, apoptosis, cellular differentiation, embryogenesis and morphogenesis, wound healing, immune responses, etc. However, the MMPs participate practically in all types of pathological conditions; for example, they are secreted and activated in diseases such as periodontal disease, rheumatoid arthritis, asthma, liver fibrosis, autoimmune diseases, and cancer [4].
MMPs are synthesized in non-active form as proenzymes (zymogenes, proMMP), and the MMP activity is regulated via gene expression, proenzyme activation, and endogenous tissue inhibitors, called tissue inhibitors of matrix metalloproteinases (TIMPs). There are four types of TIMPs that are present in humans (TIMPs 1–4). Together with MMPs, TIPMs play a major role in the remodeling of ECM and in the replenishing of its components [5][6].
Several reports have proven the important role of both MMPs and TIPMs in oral health, describing the implication of MMPs/TIPMPs balance in the initial development of the tooth and during the enamel maturation. They are also found in both intact and carious dentin, as well as in the pulp and in the saliva [7]. MMPs are expressed by different types of cells in the oral cavity. For example, in an early report from 1994, MMP-9 was mainly detected in gingival keratinocytes, while MMP-2 was expressed by gingival and granulation tissue fibroblasts [8]. Later, Tervahartiala et al., have described expressions of MMP-2, -7, -8, and -13 in gingival sulcular epithelium. In addition, MMP-7 and -13 were also found to be secreted by fibroblasts and macrophages and MMP-8 by neutrophils [9].
2. MMPs and MMP-8 in Periodontal Diseases
Periodontitis is a multifactorial disease that causes soft tissue and bone loss. Severe periodontitis affects 740 million people worldwide and is the sixth most prevalent disease. The diagnosis is based on the evaluation of the standard clinical parameters [3].
The main initiator of this chronic inflammatory disease is the interaction between the pathogenic biofilm in the subgingival and the aberrant host immune response [10]. Evidence suggests that MMPs and their tissue inhibitors (TIMPs) play an important role in tissue remodeling and tissue destruction in general and in dental tissues as periodontal tissues in particular [7][11]. Periodontal inflammation is associated with the disruption of the balance between MMPs and TIMPs [12] (Figure 1).
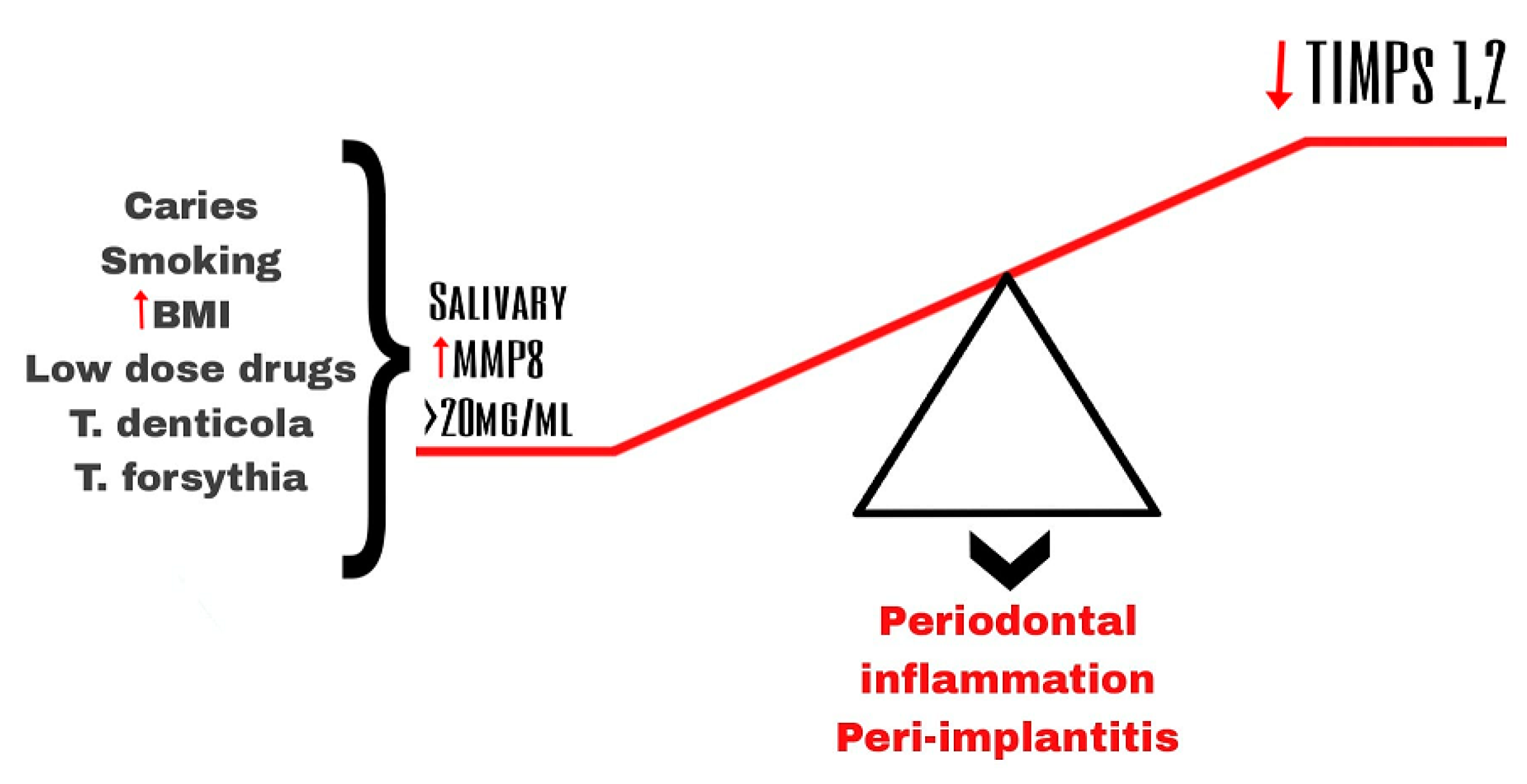
Figure 1. Imbalance of salivary MMP-8 and TIPMs leading to periodontal inflammation and peri-implantitis.
Stimulation of the host cells by the pathogens from the dental plaque is considered a type of indirect mechanism of destruction of the tissues in periodontitis [5] (Figure 1).
Pathogens such as Treponema denticola (T. denticola), Tannerella forsythia (T. forsythia), and Porphyromonas gingivalis (P. gingivalis), which are the main components of pathogenic biofilm found in the gingival crevicular fluid and plague, induce a cascade which leads to the increased levels of an active form of several MMPs [4][13][14]. Several studies have demonstrated that those pathogens activate the secretion (e.g., MMP-2, MMP-9) and especially the activation of MMPs (e.g., MMP-8) both by bacterial-derived protease (a serine-type protease) and by the oxidative stress and release of myeloperoxidase (MPO) caused by the respiratory burst during the neutrophil phagocytosis [4].
In periodontal diseases, special attention has been paid to three collagenases (MMP-1, MMP-8, and MMP-13) and to the gelatinases (MMP-2 and MMP-9) because the major component of the ECM is collagen type I. All other MMPs (MMP-7, -12, -14) and proteases have relatively moderate effects in periodontitis [4].
Recently, MMP-8 has been considered to be one of the most promising biomarkers for early detection of periodontitis and for its progression and prognosis of treatment [3][4]. Elevated salivary and gingival crevicular fluid (GCF) MMP-8 levels have been reported in patients with initial and chronic periodontitis and in those with periodontitis linked to diabetes [3][15], while the antibiotic and/or scaling and root planning treatment, as well as the application of MMP inhibitory adjuvant medicines, have been shown to lead to the reduction of the level of MMP-8 [4][16][17][18]. For example, Yakov et al. reported that the persistent increase in MMP-8 in the gingival crevicular fluid is an indicator of a high risk of poor response to periodontal therapy [13]. Sensitive MMP-8-based assays of saliva, applied as chair-side kits, have been recently created [3]. These assays effectively distinguish clinically healthy sites and gingivitis from periodontitis and could also be applicable in monitoring the treatment of patients with chronic periodontitis [18]. Thus the introduction of that method for periodontal disease testing would give an advantage not only for the diagnosis but also for the identification of susceptible individuals and the prognosis of treatment [3][19].
Factors determining the susceptibility for periodontitis are some functional variants of the genes encoding the MMPs [20][21]. Variations in the MMP8 gene that have been mostly investigated in association with periodontitis are MMP8 −799 C > T (rs11225395) +17 C/G (rs2155052) and −381A/G (rs11225395) (Table 1).
Table 1. Observations from studies concerning MMP8 −799 C > T (rs11225395) +17 C/G (rs2155052) and −381A/G (rs11225395) polymorphisms in periodontitis.
| Polymorphism in MMP8 |
Population | Disease | Patients/Controls | Observation |
|---|---|---|---|---|
| −799 (C > T) | Taiwan | AgP + CP | 96 + 361/106 | Increased risk for AgP (p = 0.04) and CP (p = 0.007) in carriers of T allele [22]. |
| −799 (C > T) | No differences in allele (p = 0.06) and genotype (p = 0.280 distributions. No associations with particular periodontal pathogen [23] | |||
| Czech | CP | 341/278 | ||
| +17 (C > G) | Czech | CP | 341/278 | No differences in allele (p = 0.38) and genotype (p = 0.09) distributions. No associations with particular periodontal pathogen [23] |
| −799C>T/+17C > G haplotypes |
Czech | CP | 341/278 | -799T/+17C haplotype is associated with 1.273-fold risk of CP (p = 0.038) [23] |
| −799 (C > T) | Turkish | GAgP | 100/267 | T allele (p < 0.0001) and T allele genotypes (CT + TT, p < 0.0001) were more common in GAgP determining 2.878-; 6.76-fold higher risk of GAgP compared to the wild C allele and CC genotype [24]. |
| +17 (C > G) | Turkish | GAgP | 100/267 | No differences in allele (p = 0.290) and genotype (p = 0.581) distributions [24] |
| −381 (A > G) | Turkish | GAgP | 100/267 | G allele (p = 0.027) and G allele genotypes (AG + GG, p = 0.015) were less common in GAgP determining 1.5- and 2.27-fold lower risk of GAgP (OR = 0.664 and OR = 0.44) compared to the wild A allele and AA genotype [24] |
CP—chronic periodontitis; AgP—aggressive periodontitis; GAgP—generalized aggressive periodontitis.
As it is shown in Table 1, there are quite a few studies for MMP8 gene variants in the development of periodontitis, and the results show that polymorphisms in the promoter regions (−799 C > T, −381 A > G) might be of importance as predisposing factors both for chronic and aggressive periodontitis [22][24]. The results of the two meta-analyses published lead to similar conclusions suggesting that the variant T allele of MMP8 −799 C > T SNP is associated with an increased risk of periodontitis in four genetic models [21][25]. When the meta-analysis was performed in subgroups, it proved that the increased risk was mainly valid for Asians, for chronic periodontitis, and non-smokers [25].
There are also a few other MMPs evaluated in saliva and GCF and in periodontitis gingiva in patients with periodontitis. The immunohistochemically labeled cells for MMP-13 and for MMP-8 were higher in density in periodontitis gingiva when compared with healthy control tissue (p < 0.01). In periodontal diseases, gingival sulcular epithelium expresses several, rather than a single, collagenolytic MMPs, and this proteolytic cascade is evidently responsible for the tissue destruction characteristic of adult and juvenile periodontitis [9].
Besides MMP-8, decreased levels in gingival crevicular fluids after the effective treatment of periodontitis have also been observed for MMP-1, -9, -12, and -13 [4][26][27]. Indeed, the analyses of GCF from 29 African-American individuals diagnosed with localized aggressive periodontitis treated with full-mouth scaling and root planning and systemic antibiotics have proven that the levels of MMP-1, -8, -9, -12 and -13 were significantly reduced up to 6 months after the beginning of therapy and correlated positively with some clinical parameters as the pocket depth [26]. Even more, MMP-9 found in the saliva is shown to be a more sensitive biomarker during orthodontic treatment, which is promising for the decreasing of periodontal hazards during such manipulations [4].
In addition, significant associations were found between MMP-8 and MMP-9 activities in gingival crevicular fluid and the severity of the periodontal disease, together with negative correlations with TIMP-1 and TIMP-2 levels. This means that there is a dynamic in the balance between the active MMPs and their endogenous inhibitors, TIMPs, and suggests that MMP inhibitors could be a part of an innovative therapy against the effects of MMP on periodontal tissues [2]. Such MMP inhibitory effect is expressed by subantimicrobial doses of doxycycline, which have been approved as adjuvant therapy for treating periodontitis [28].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11061514
References
- Schubert-Unkmeir, A.; Konrad, C.; Slanina, H.; Czapek, F.; Hebling, S.; Frosch, M. Neisseria meningitidis induces brain microvascular endothelial cell detachment from the matrix and cleavage of occludin: A role for MMP-8. PLoS Pathog. 2010, 6, e1000874.
- Checchi, V.; Maravic, T.; Bellini, P.; Generali, L.; Consolo, U.; Breschi, L.; Mazzoni, A. The Role of Matrix Metalloproteinases in Periodontal Disease. Int. J. Environ. Res. Public. Health 2020, 17, 4923.
- Gul, S.S.; Abdulkareem, A.A.; Sha, A.M.; Rawlinson, A. Diagnostic Accuracy of Oral Fluids Biomarker Profile to Determine the Current and Future Status of Periodontal and Peri-Implant Diseases. Diagnostics 2020, 10, 838.
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806.
- Franco, C.; Patricia, H.R.; Timo, S.; Claudia, B.; Marcela, H. Matrix Metalloproteinases as Regulators of Periodontal Inflammation. Int. J. Mol. Sci. 2017, 18, 440.
- Wang, X.; Rojas-Quintero, J.; Wilder, J.; Tesfaigzi, Y.; Zhang, D.; Owen, C.A. Tissue Inhibitor of Metalloproteinase-1 Promotes Polymorphonuclear Neutrophil (PMN) Pericellular Proteolysis by Anchoring Matrix Metalloproteinase-8 and -9 to PMN Surfaces. J. Immunol. 2019, 202, 3267–3281.
- Goldberg, M. Dental Tissues Remodeling by Matrix Metalloproteinases (MMPS) and Tissues Inhibitors of MMPS (TIMPS). SVOA Dent. 2022, 3, 203–212.
- Makela, M.; Salo, T.; Uitto, V.J.; Larjava, H. Matrix metalloproteinases (MMP-2 and MMP-9) of the oral cavity: Cellular origin and relationship to periodontal status. J. Dent. Res. 1994, 73, 1397–1406.
- Tervahartiala, T.; Pirila, E.; Ceponis, A.; Maisi, P.; Salo, T.; Tuter, G.; Kallio, P.; Tornwall, J.; Srinivas, R.; Konttinen, Y.T.; et al. The in vivo expression of the collagenolytic matrix metalloproteinases (MMP-2, -8, -13, and -14) and matrilysin (MMP-7) in adult and localized juvenile periodontitis. J. Dent. Res. 2000, 79, 1969–1977.
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S74–S84.
- de Morais, E.F.; Pinheiro, J.C.; Leite, R.B.; Santos, P.P.A.; Barboza, C.A.G.; Freitas, R.A. Matrix metalloproteinase-8 levels in periodontal disease patients: A systematic review. J. Periodontal Res. 2018, 53, 156–163.
- Letra, A.; Silva, R.M.; Rylands, R.J.; Silveira, E.M.; de Souza, A.P.; Wendell, S.K.; Garlet, G.P.; Vieira, A.R. MMP3 and TIMP1 variants contribute to chronic periodontitis and may be implicated in disease progression. J. Clin. Periodontol. 2012, 39, 707–716.
- Yakob, M.; Kari, K.; Tervahartiala, T.; Sorsa, T.; Soder, P.; Meurman, J.H.; Soder, B. Associations of periodontal microorganisms with salivary proteins and MMP-8 in gingival crevicular fluid. J. Clin. Periodontol. 2012, 39, 256–263.
- Yakob, M.; Meurman, J.H.; Sorsa, T.; Söder, B. Treponema denticola associates with increased levels of MMP-8 and MMP-9 in gingival crevicular fluid. Oral. Dis. 2013, 19, 694–701.
- Sapna, G.; Gokul, S.; Bagri-Manjrekar, K. Matrix metalloproteinases and periodontal diseases. Oral. Dis. 2014, 20, 538–550.
- Emingil, G.; Atilla, G.; Sorsa, T.; Luoto, H.; Kirilmaz, L.; Baylas, H. The effect of adjunctive low-dose doxycycline therapy on clinical parameters and gingival crevicular fluid matrix metalloproteinase-8 levels in chronic periodontitis. J. Periodontol. 2004, 75, 106–115.
- Konopka, L.; Pietrzak, A.; Brzezinska-Blaszczyk, E. Effect of scaling and root planing on interleukin-1β, interleukin-8 and MMP-8 levels in gingival crevicular fluid from chronic periodontitis patients. J. Periodontal. Res. 2012, 47, 681–688.
- Honibald, E.N.; Mathew, S.; Padmanaban, J.; Sundaram, E.; Ramamoorthy, R.D. Perioceutics: Matrix metalloproteinase inhibitors as an adjunctive therapy for inflammatory periodontal disease. J. Pharm. Bioallied Sci. 2012, 4, 0975–7406.
- Sorsa, T.; Gursoy, U.K.; Nwhator, S.; Hernandez, M.; Tervahartiala, T.; Leppilahti, J.; Gursoy, M.; Könönen, E.; Emingil, G.; Pussinen, P.J.; et al. Analysis of matrix metalloproteinases, especially MMP-8, in gingival creviclular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontology 2000, 70, 142–163.
- Li, W.; Zhu, Y.; Singh, P.; Ajmera, D.H.; Song, J.; Ji, P. Association of Common Variants in MMPs with Periodontitis Risk. Dis. Markers 2016, 2016, 1545974.
- da Silva, M.K.; de Carvalho, A.C.G.; Alves, E.H.P.; da Silva, F.R.P.; Pessoa, L.D.S.; Vasconcelos, D.F.P. Genetic Factors and the Risk of Periodontitis Development: Findings from a Systematic Review Composed of 13 Studies of Meta-Analysis with 71,531 Participants. Int. J. Dent. 2017, 2017, 1914073.
- Chou, Y.H.; Ho, Y.P.; Lin, Y.C.; Hu, K.F.; Yang, Y.H.; Ho, K.Y.; Wu, Y.M.; Hsi, E.; Tsai, C.C. MMP-8 -799 C>T genetic polymorphism is associated with the susceptibility to chronic and aggressive periodontitis in Taiwanese. J. Clin. Periodontol. 2011, 38, 1078–1084.
- Izakovicova Holla, L.; Hrdlickova, B.; Vokurka, J.; Fassmann, A. Matrix metalloproteinase 8 (MMP8) gene polymorphisms in chronic periodontitis. Arch. Oral. Biol. 2012, 57, 188–196.
- Emingil, G.; Han, B.; Gurkan, A.; Berdeli, A.; Tervahartiala, T.; Salo, T.; Pussinen, P.J.; Kose, T.; Atilla, G.; Sorsa, T. Matrix metalloproteinase (MMP)-8 and tissue inhibitor of MMP-1 (TIMP-1) gene polymorphisms in generalized aggressive periodontitis: Gingival crevicular fluid MMP-8 and TIMP-1 levels and outcome of periodontal therapy. J. Periodontol. 2014, 85, 1070–1080.
- Weng, H.; Yan, Y.; Jin, Y.H.; Meng, X.Y.; Mo, Y.Y.; Zeng, X.T. Matrix metalloproteinase gene polymorphisms and periodontitis susceptibility: A meta-analysis involving 6162 individuals. Sci. Rep. 2016, 6, 24812.
- Goncalves, P.F.; Huang, H.; McAninley, S.; Alfant, B.; Harrison, P.; Aukhil, I.; Walker, C.; Shaddox, L.M. Periodontal treatment reduces matrix metalloproteinase levels in localized aggressive periodontitis. J. Periodontol. 2013, 84, 1801–1808.
- Cifcibasi, E.; Kantarci, A.; Badur, S.; Issever, H.; Cintan, S. Impact of metronidazole and amoxicillin combination on matrix metalloproteinases-1 and tissue inhibitors of matrix metalloproteinases balance in generalized aggressive periodontitis. Eur. J. Dent. 2015, 9, 53–59.
- Luizon, M.R.; de Almeida Belo, V. Matrix metalloproteinase (MMP)-2 and MMP-9 polymorphisms and haplotypes as disease biomarkers. Biomarkers 2012, 17, 286–288.
This entry is offline, you can click here to edit this entry!
