Glucagon-like peptide-1 receptor agonists (GLP1-RA) and dipeptidyl peptidase-4 inhibitors (DPP4i) were recently introduced as novel classes of drugs for treating patients with type 2 diabetes (T2D). However, several cardiovascular preclinical and clinical studies, originally designed to investigate their safety in clinical practice, early reported an impressive and effective performance of these agents in reducing cardiovascular (CV) risk, and thus attenuated incidence of acute myocardial infarction or stroke in patients with T2D receiving these drugs associated with lower cardiovascular mortality [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13]. Following this evidence, both cardiological and endocrinological guidelines [
14,
15] now recommend GLP-1 RA for managing patients with both T2D and high atherosclerotic cardiovascular risk, moving from a merely glucometabolic approach to a more comprehensive strategy focusing on cardiovascular prevention in the setting of T2D.
In particular, the 2019 European Society of Cardiology (ESC) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases, developed in collaboration with the European Association for the Study of Diabetes (EASD), advise GLP1-RAs in patients with T2D and CV disease or at high CV risk to reduce cardiovascular events (class of recommendation I, level of evidence A) [
15]. More recently, the 2021 ESC Guidelines on cardiovascular disease prevention confirmed this indication, suggesting GLP1-RAs in individuals with T2D and atherosclerotic cardiovascular disease (ASCVD) to reduce CV and cardiorenal outcomes (class of recommendation I, level of evidence A) [
14].
Although these drugs revolutionized diabetes therapy, their use was limited by several contraindications that should be considered before prescription, such as pancreatitis, pregnancy or breastfeeding, history of previous hypersensitivity reactions, personal or family history of medullary thyroid cancer, and multiple endocrine neoplasias (MEN) [
16]. Lastly, administering these drugs by injections may represent another drawback since most patients prefer an oral therapeutic regimen. Conversely, these agents rarely cause hypoglycaemia, representing really safe drugs.
2. Physiological Mechanisms of Incretins
Atherosclerosis is a multifactorial process characterized by forming fibrofatty lesions within the arterial wall and is considered the leading cause of death worldwide [
18]. Improvement in treatment and prevention is crucial, especially in patients with T2D, a clinical syndrome expected to affect 783.2 million people by 2045 [
19]. Therefore, the treatment guidelines for T2D patients recommend a patient-tailored approach based on lifestyle modifications and the choice of optimal therapeutic option. An ideal anti-diabetic drug should have the following characteristics: significant impact on weight and cardiovascular comorbidities, low risk of hypoglycemia and adverse events, and, last but not least, low costs. Even if no optimal medication exists, incretins represent one of the most attractive and promising options [
20]. The “incretin effect” indicates the amplification of pancreatic insulin secretion induced by these gastrointestinal tract-released hormones [
21]. Incretins were demonstrated to reduce glucagon concentrations, improve insulin sensitivity, and slow down gastric filling in diabetic patients, with decreased free fatty acid concentrations and body weight. Moreover, beyond glycemic control, incretins protect the cardiovascular system [
22]. The glucose-dependent insulinotropic polypeptide (GIP), a 42 amino acid hormone, and glucagon-like peptide-1 (GLP-1), a 31 amino acid hormone, are the most critical studied incretins. They bind to distinct G-protein-coupled receptors highly expressed on pancreatic β-cell surfaces. More specifically, while GLP-1 is secreted by L-cells in the ileum, colon, and rectum, GIP is released from K-cells located predominately in the duodenum and proximal gut after feeding. GIP circulates in 10-fold higher concentrations than GLP-1, whereas GLP-1 appears more potent than GIP. The two hormones are secreted in parallel but are not stored in the same cytoplasmic granules [
23]. GIP and GLP1 can be degraded by DPP-4, an amino-peptidase transmembrane protein with a large extracellular domain and a flexible segment anchored in the cell membrane expressed in most cell types. The enzyme is responsible for cleaving and inactivating both two peptides at one of the last alanine residues [
24]. Inhibition of DPP-4 and the use of injectable GLP-1RA are the two strategies for potentiate incretin receptor signaling [
23]. However, studies on GIP monotherapy were unsuccessful; this could be explained by the fact that, in diabetic patients, the endocrine pancreas remains responsive to GLP-1, but it is no longer responsive to GIP, so this must represent the most likely reason for the reduced incretin action of this hormone [
25].
2.1. Acute and Chronic Effects of GLP-1 on Pancreatic β Cells
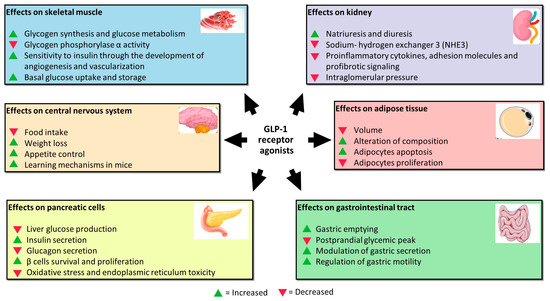
GLP-1 exerts acute and chronic functions on pancreatic cells by binding to its receptor (
Figure 1). Acutely, it triggers most insulin release from these cells in a glucose-dependent manner. Hence, glucose enters the pancreatic β-cells through glucose transporter-2 (GLUT2). After phosphorylation, glycolysis, and the mitochondrial tricarboxylic acid (TCA) cycle, glucose determines adenosine triphosphate (ATP) production. Increased intracellular ATP concentration leads to K+ ATP-dependent channel closures with consequent accumulation of K+ ions and membrane depolarization. Thus, cell membrane depolarization causes voltage-dependent Ca2+ channel activation, the influx of Ca2+ ions, and exocytosis of a sub-pool of insulin granules, which contains ∼1–5% of available insulin. This initial rapid process is followed by the remaining entire insulin release mediated by GLP-1 binding to its receptor (GLP-1R), as previously mentioned, allowing the release of ∼95–99% of insulin granules [
26]. GLP-1R is a G protein-coupled receptor whose activation induces a rapid increase in c-adenosine monophosphate (cAMP), protein kinase A (PKA), and exchange protein activated directly by cAMP (EPAC) upregulation and increased glucose-dependent insulin release [
27]. Further to the stimulation of insulin secretion, the chronic effects of GLP-1RA on pancreatic β cells also consist of the deceleration of mass reduction.
Figure 1. Effects of GLP-1RA on pancreatic β cells and other cell lines.
Moreover, GLP-1RA-mediated cAMP elevation is responsible for oxidative stress reduction and trough activation of anti-apoptotic genes for pancreatic β cells survival. The latter process is also stimulated by GLP-1RA-induced secretion of insulin-like growth factor-2 (IGF-2) and expression of its receptor (IGF1-R) in β cells. The IGF-2 binding to its receptor determines an autocrine loop that protects β-cells against apoptosis, inducing proliferation [
28,
29].
2.2. Effects of GLP-1 on Other Cell Lines
GLP-1 performs its function by acting not only on pancreatic β cells, but also on other cell lines (
Figure 1). Studies assessing GLP-1RA extra-pancreatic effects reveal that exposure of skeletal muscle cells to these agents increases glycogen synthesis, glycogen synthase activity, and glucose metabolism and inhibits glycogen phosphorylase α activity involved in the breaking up of glycogen in glucose subunits [
30]. Moreover, skeletal muscles exposed to increased incretin levels become more sensitive to insulin through more developed angiogenesis and vascularization. Indeed, GLP-1 binds to its receptor, abundantly expressed in endothelial cells, causing microvasculature recruitment in skeletal muscles and better muscle perfusion. Muscle perfusion is also favored via PKA-mediated endothelial nitric oxide synthase (eNOS) activation, which induces vasodilation and consequently increases insulin delivery [
31]. These processes allow skeletal muscles to increase basal glucose uptake and storage.
GlP-1 exerts a protective effect on the kidney. It is responsible for inhibiting sodium–hydrogen exchanger 3 (NHE3), an antiporter located on the epithelial cells of the proximal tube that imports sodium ions, simultaneously ejecting hydrogen ions in the proximal tubule lumen. NHE3 inhibition increases natriuresis and diuresis [
32,
33,
34].
The mechanisms of this renal function gain may be direct and indirect. In addition to natriuresis, the first ones include the inhibition of proinflammatory cytokines, adhesion molecules, and profibrotic signaling, as well as the reduction in intraglomerular pressure through the inhibition of protein kinase-C (PKC) and the activation of PKA. The indirect mechanisms regard the benefit that GLP-1R agonists exert on other tissues, including improvement in blood pressure, glucose homeostasis, weight loss, and insulin levels that are beneficial for glomerular filtrate [
35].
Furthermore, GLP-1 receptors were demonstrated to be located in many areas of the central nervous system; in particular, in those involved in appetite and gastric motility regulation [
36]. GLP-1 is not only produced by alfa and L-cells, but also by neurons. It was proved that neuronally produced GLP-1 is transported to the axon terminals and stored in synaptic vesicles until release into the synaptic cleft, or in case of extra-synaptic release, into the brain parenchyma. Recent pre-clinical studies reported that administering GLP-1RA reduces food intake, determining weight loss in animal models [
37]. The underlying mechanism is that L-cells-derived GLP-1 passes through the blood–brain barrier by directly affecting the receptors in the hypothalamic areas responsible for appetite control. GLP-1 is also released into the interstitial space near the site of its synthesis (ileum and colon) and then diffuses locally to act on vagal nerve endings embedded into the gut mucosa [
38]. Moreover, the administration of GLP-1 in mice improves learning mechanisms, and the deletion of the gene encoding for GLP-1R is associated with neuron degeneration. Because of this, GLP-1RA was proposed as adjuvant therapy in the treatment of neurodegenerative pathologies such as Alzheimer with encouraging results [
39].
In visceral adipose tissue, GLP-1 was demonstrated to reduce volume and alter the composition in vivo [
40]. GLP-1 exerts this effect through activation of extracellular signal-regulated kinase (ERK), PKC, and AKT pathways with consequent increased adipocytes apoptosis and reduced pre-adipocytes proliferation [
27].
After all, GLP-1 acts directly at the gastrointestinal level, inhibiting gastric emptying and postprandial glycaemic peak reduction due to the slower transit of food from the stomach to the small intestine. The mechanisms are still unknown, but GLP-1R is expressed on the parietal cells of the stomach to indicate a direct action of GLP-1 on their secretion. In addition, vagal denervation was shown to abolish the inhibitory effect of GLP-1 on gastric emptying, suggesting that GLP-1 acts through receptors expressed on vagal fibres that regulate gastric motility [
41].