Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The incidence of chronic maternal disease ranges from 10 to 30% of pregnancies worldwide. Several epidemiological studies in mothers with chronic diseases have mainly focused on the risk for adverse obstetric outcomes. Evidence from these studies supports a correlation between maternal chronic conditions and adverse perinatal outcomes, including increased risk for preeclampsia, cesarean section, preterm birth, and admission in the Neonatal Intensive Care Unit (NICU). However, there is a knowledge gap pertaining to the management of these women during lactation.
- breastfeeding
- maternal chronic disease
- neonates
1. Introduction
Breastfeeding is the best and most natural nutrition for infants. Through breastfeeding, infants are offered all the necessary nutrients and elements for their optimal growth and development. The World Health Organization, Unicef, and the American Academy of Pediatrics recommend exclusive breastfeeding for the first 6 months of life and continuation of breastfeeding (after introduction of solid foods at 6 months) until the first year of life and for as long as the mother and child desire [1][2][3]. There is indisputable evidence in the literature regarding breastfeeding benefits for the infant, the mother, the family, and the society, in general [4]. Maternal milk contains the ideal qualitative and quantitative composition for optimal neonatal growth. Breastfeeding contributes to the smooth physical and psychological development of the infant, conferring short-term as well as long-term benefits. First of all, breastfed infants have a decreased risk of childhood mortality [5][6][7]. Research has revealed that infants who have been breastfed for less than two months and those who are partially or not breastfed have a higher mortality risk compared to exclusively breastfed infants [8][9]. Breastfeeding for more than six months protects against obesity, diabetes, asthma, cardiac conditions, and increases final height [10][11][12][13][14]. Rich-Edwards et al. [14] investigated the association between breastfeeding and cardiac conditions and suggested that breastfed infants may present a lower risk of ischemic cardiovascular disease in adulthood. Additionally, breastfed children seem to have lower risk of developing certain types of childhood cancer, including leukemia and lymphomas [15][16]. Breastfeeding positively impacts cognitive, emotional, and social development of the infant [17][18]. Neonatal mortality and morbidity is reduced in breastfed neonates, in particular preterm newborns. Breastfeeding fortifies the immune system, promoting immune maturation and protecting infants against infections. Breastmilk interacts with gut microbiota and, to a degree, shapes microbiome colonization, with possible effects on long-term programming [19][20].
Breastfeeding contributes to the regular physical and psychological development of the infant, with short-term and long-term advantages. The majority of mothers are able to breastfeed and entitled to it, providing they are offered accurate information and are supported by family, healthcare system, and society.
The presence of a chronic disease is increasingly common in pregnant women, with a frequency of up to 10–30% [21][22]. The prevalence of chronic maternal disease is rising in the last decades in the developed world, with a reported increase from 4% in 1989 to 16% in 2013 [21]. This trend is possibly explained by the rise in disease rates in the general population, the increase in mean childbearing age of women, and the medical progress in assisted reproduction. Research in mothers with chronic diseases has mainly focused on their risk for adverse obstetric outcomes. Evidence from these studies supports a correlation between maternal chronic conditions and adverse perinatal outcomes, including increased risk for preeclampsia, cesarean section, preterm birth, and admission in the Neonatal Intensive Care Unit (NICU) [23][24][25][26][27]. However, there is a knowledge gap pertaining to the management of these women during lactation.
2. Autoimmune Diseases and Breastfeeding
Advantages of breastfeeding in mothers with autoimmune diseases are outlined in the literature. The high prevalence of autoimmune conditions among women indicates the crucial role of gender and hormonal implication in the pathogenesis of these diseases. Evidence suggests a relationship between prolactin and autoimmune diseases, in particular systematic lupus erythematosus (SLE), rheumatoid arthritis (RA), and peripartum cardiomyopathy (PPCM) [28][29][30][31][32][33]. Prolactin is mainly produced in the pituitary gland and its role is not only to stimulate the growth of the mammary gland and the production of milk during lactation, but also to modify the maternal behavior. Genes coding for prolactin are located in chromosome 6, close to HLA-DRB1. Polymorphisms of the human prolactin gene may affect the pathogenesis of autoimmune conditions [29][34]. Elevated prolactin levels may interfere with B-cell tolerance through various mechanisms [28][35], while prolactin induces the expression of IL-2 receptor and the production of IFN-γ and IL-1 (Figure 1). It modifies the maturation of dendritic and thymus cells, leading to IFN-α production and enhancement of pro-B-cells generation.
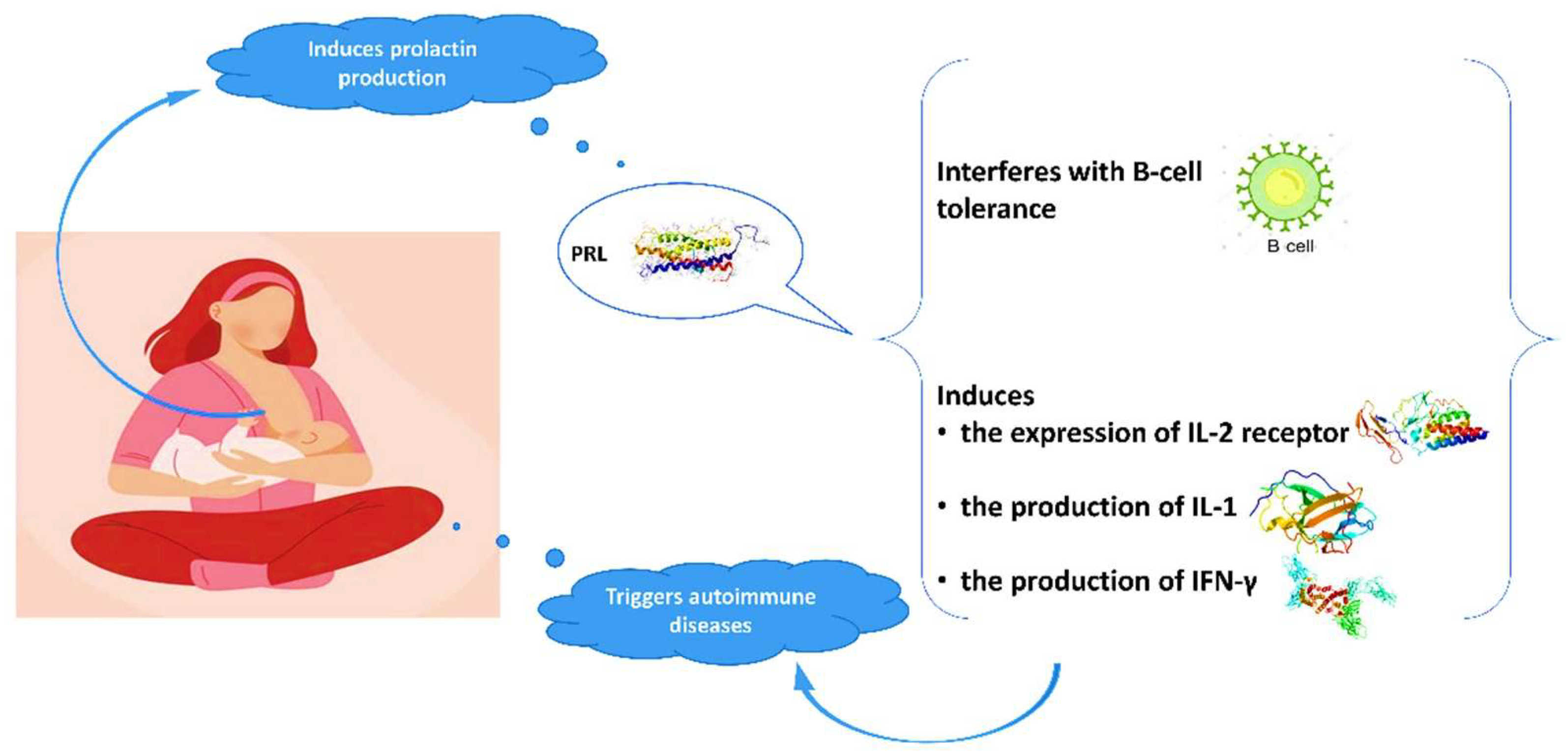
Figure 1. Interaction of prolactin with autoimmune system.
2.1. Systematic Lupus Erythematosus and Breastfeeding
Systematic lupus erythematosus (SLE) is a chronic, multisystemic, inflammatory disease of autoimmune etiology, commonly presented in young women of reproductive age. Prolactin has been found to be implicated in the pathogenesis of antiphospholipid syndrome and the observed impaired fertility [36][37][38]. Recently, Song et al. [31], in a meta-analysis, revealed a significantly positive correlation between prolactin and SLE activity. In a large, cohort study by Orbach et al. [39], SLE patients with hyperprolactinemia presented with significantly more episodes of pleuritis, pericarditis, and peritonitis, and had more frequently anemia and proteinuria compared to patients with normal prolactin levels. The authors concluded that dopamine agonists could be a potential treatment for SLE patients with hyperprolactinemia. In other studies, treatment with bromocriptine has reduced disease activity, and therapy cessation was associated with SLE flares [36][37]. Current evidence supports the benefits of treatment with bromocriptine in refractory SLE or in the prevention of flares after labor [37]. Conclusively, these findings question whether mothers with SLE should breastfeed.
Contrary to the numerous prospective and retrospective studies published on pregnancy outcomes of women with SLE [40][41], data regarding breastfeeding are currently scarce. Breastfeeding rates and duration seem to be decreased in SLE patients. Orefice et al. [42] in a cohort study reported that the vast majority of mothers with SLE (96.5%) were planning to breastfeed during pregnancy and 71.9% did breastfeed. However, half of these patients ceased breastfeeding after 3 months. Additionally, factors including cesarean section, preterm birth, intrauterine growth restriction (IUGR), and disease flares were positively correlated with non-breastfeeding. Also, a relationship between treatment with hydroxychloroquine (HCQ) and delayed breastfeeding cessation was reported for the first time. In subsequent studies, HCQ has shown reduction in disease relapse risk during and after pregnancy [43][44], decreased rates of recurrent neonatal lupus and improvement of labor outcome [45][46]. In another study, Noviani et al. reported that 49% of participating SLE patients decided to breastfeed [47]. This rate was not significantly affected by socioeconomic factors. Furthermore, disease activity after labor, full-term labor, breastfeeding education and planning were positively correlated with breastfeeding. Transfer of HCQ, azathioprine, methotrexate, and prednisone to maternal milk seems very limited and all drugs are compatible with breastfeeding [47]. Acevedo et al. [48] recorded reduced breastfeeding rates and duration in SLE patients: they breastfed their children half of the time that the mothers without SLE did (6 months vs. 12 months, respectively). The initiation of a new treatment was the main reason for breastfeeding cessation in spite of the fact that these drugs were low risk for breastfeeding. Breastfeeding duration could be improved by enhancing the level of information provided to patients. Complications during the postnatal period were mainly responsible for not initiation of breastfeeding. HCQ is compatible with breastfeeding according to the American Academy of Pediatrics (AAP), and most SLE specialists recommend continuation of breastfeeding in SLE patients receiving antimalarial medicines [49][50]. Prednisone and ibuprofen in low doses are also acceptable options during pregnancy, while data on the use of other medicines for the treatment of SLE in pregnancy and lactation are limited. Current recommendations advocate for the initiation of HCQ when pregnancy is scheduled and the continuation of the drug throughout pregnancy and lactation [51].
2.2. Rheumatoid Arthritis and Breastfeeding
High prolactin levels may result either in autoimmune disease presentation in mothers with predisposition or in flares in patients with existing conditions [52]. Risk for RA onset increases during the postpartum period, particularly after the first pregnancy [53]. Women who developed RA within 12 months of the first pregnancy were five times more likely to have breastfed, while breastfeeding rates sharply declined in a subsequent pregnancy [54]. Barrett et al. [55] compared disease activity during and for 6 months after pregnancy between 49 patients who did not breastfeed, 38 who breastfed for the first time and 50 who had previous breastfeeding experience. Following adjustment for possible confounding factor, including treatment, patients who were breastfeeding for the first time showed increased disease activity 6 months after labor, indicated by self-reported symptoms, number of affected joints, and C-reactive protein levels, suggesting that this flare could be caused by breastfeeding. Brennan and Silman [54] investigated whether the presentation of RA after labor could be attributed to breastfeeding. Through a media campaign, the authors interviewed 187 women who presented RA within 12 months of labor and compared their breastfeeding history with that of 149 women of similar age selected from the patient registers of a nationwide group of general practitioners. In total, 88 women developed RA after their first pregnancy, and 80% of them breastfed. This rate was higher than the prevalence of breastfeeding (50%) in the 129 controls (adjusted odds ratio (aOR) 5.4, 95% confidence intervals (CI) 2.5–11.4). The risk for RA development was less increased following breastfeeding in a second (OR 2.0) and not increased in a third pregnancy (OR 0.6). More recently, Eudy et al. [56] in a cross-sectional study reported that most women with RA breastfed and were regularly receiving treatment during lactation. Disease activity seemed to worsen, in particular for the patients who did not receive treatment during lactation, while improvement was only observed in women who followed treatment during breastfeeding. Ince-Askan et al. [57] in a prospective cohort study concluded that only 4% of mothers with arthritis exclusively breastfed until 26 weeks compared to 25% of the general population.
2.3. Idiopathic Inflammatory Bowel Diseases and Breastfeeding
Idiopathic inflammatory bowel diseases (IBDs) are chronic intestinal disorders usually diagnosed during the second and third decades of life. The effect of pregnancy on the course of disease varies; the majority of patients remain in remission, while a few of them improve probably due to generalized immunosuppression during gestation. Contrarily, 1/3 of patients deteriorate [58][59]. Although the exact mechanisms explaining aggravation in pregnancy are not known, it is speculated that the cause may be the lack of maternal- fetal immunocompatibility [60]. Consequently, women with IBD may present with disease activation and need treatment at conception, pregnancy, or labor. Women with IBD are at increased risk for spontaneous abortions, preterm labor, and low birth weight neonates [59][61]. Some researchers suggest an increased risk of chromosomal disorders (although the role of disease activity relative to that of the drug exposures has not been elucidated) and adverse perinatal outcomes in patients with IBD [59][62]. Breastfeeding rates in women with IBD range among studies. Dotan et al. [62] reported that mothers with IBD breastfed less frequently. Approximately 1/3 of them did not breastfeed at all compared to 1/5 of healthy controls (p < 0.005), and short-term and long-term breastfeeding were also less common in mothers with IBD. Moreover, mothers who received treatment with immunomodulators and steroids had significantly lower breastfeeding rates in comparison to women who were only administered 5-ASA. In a study by Moffatt et al. [63], 83.3% of IBD patients began to breastfeed compared to 77.1% of the general population (p > 0.05). With regards to breastfeeding duration, 56.1% of IBD patients vs. 44.4% of the general population breastfed for more than 24 weeks (p = 0.02) [63]. The rate of disease flare during the first year after labor was 26% for breastfeeding and 29.4% for non-breastfeeding patients with Chron’s disease (CD, p = 0.76) and 29.2% for breastfeeding vs. 44.4% (p = 0.44) for non-breastfeeding women with ulcerative colitis (UC). The risk for disease flare was independent of age, disease length, and socio-economic status. The authors concluded that IBD does not seem to reduce chances of breastfeeding. Lactation is not associated with increased risk of flares; contrarily, it could be protective during the first year after labor. Kane et al. [64] studied 122 women with IBD who were asymptomatic during pregnancy. Only 44% breastfed due to doctor recommendations, fear of drug interactions, and personal choice. Of those who breastfed, 43% presented postpartum disease flare. Non-adjusted OR for disease activity in women with breastfeeding history was 2.2 (95% CI 1.2–3.9, p = 0.004). After risk stratification by disease type, OR for UC was 0.89 (95% CI 0.29–2.7, p > 0.05), and for CD it was 3.8 (95% CI 1.9–7.4, p < 0.05). Following adjustment for treatment cessation, statistical significance of OR was not retained. These results indicate that a significant number of IBD patients do not breastfeed. A relationship between breastfeeding and disease activity may be owing to IBD treatment cessation.
Breastfeeding has been associated with prevention of IBD in children [62][65][66], a benefit which should be taken into serious consideration by mothers with IBD.
2.4. Multiple Sclerosis and Breastfeeding
Multiple sclerosis (MS) is an autoimmune inflammatory disease in which sclerotic plaques are formed in the central nervous system causing neuronal demyelination and damage. MS is usually encountered in women of childbearing age [67], and although disease modifying treatments (DMTs) reduce relapse rates, none of these treatments are recommended during pregnancy or breastfeeding [68][69][70].
MS relapse rates are decreased during the last trimester of pregnancy, but they rise during the first 3 months after labor, with up to 30% of patients relapsing [71]. Postpartum relapses are associated with high risk of disability [71] and deterioration of existing disability [72]. Women are frequently faced with the dilemma of breastfeeding or not breastfeeding and re-initiating DMT after labor. Despite the many observational studies, there is no consensus to date as to whether there is a relationship between breastfeeding and postpartum relapse control [72][73][74][75]. In 2012, a meta-analysis showed that non-breastfeeding women had double the risk of postpartum relapse than breastfeeding mothers [76]. However, there is great heterogeneity among studies included in this meta-analysis, and researchers did not assess whether disease activity before pregnancy affected the findings of the study or if the results were attributed to exclusive breastfeeding and its different hormonal impact from non-exclusive breastfeeding. Krysko et al. [70], in a 2021 systematic review and meta-analysis, demonstrated that breastfeeding was correlated with lower rate of postpartum MS relapses, with this beneficial effect being greater in cases of increased disease activity and exclusive breastfeeding. For a mother with MS, and possibly mobility problems, breastfeeding advantages may help improve her quality of life and health.
This entry is adapted from the peer-reviewed paper 10.3390/nu15132822
References
- American Association of Pediatrics; Eidelman, A.I.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the Use of Human Milk. Pediatrics 2012, 129, e827–e841.
- World Health Organization (WHO). Breastfeeding. Available online: https://www.who.int/health-topics/breastfeeding#tab=tab_1 (accessed on 20 July 2022).
- UNICEF. Breastfeeding. Available online: https://data.unicef.org/topic/nutrition/breastfeeding/ (accessed on 20 July 2022).
- Benefits of breastfeeding. Nutrition in clinical care: An official publication of Tufts University. Nutr. Clin. Care 2003, 6, 125–131.
- Li, R.; Ware, J.; Chen, A.; Nelson, J.M.; Kmet, J.M.; Parks, S.E.; Morrow, A.L.; Chen, J.; Perrine, C.G. Breastfeeding and post-perinatal infant deaths in the United States, A national prospective cohort analysis. Lancet Reg. Health Am. 2022, 5, 100094.
- Sankar, M.J.; Sinha, B.; Chowdhury, R.; Bhandari, N.; Taneja, S.; Martines, J.; Bahl, R. Optimal breastfeeding practices and infant and child mortality: A systematic review and meta-analysis. Acta Paediatr. (Oslo Nor. 1992) 2015, 104, 3–13.
- Ware, J.L.; Chen, A.; Morrow, A.L.; Kmet, J. Associations Between Breastfeeding Initiation and Infant Mortality in an Urban Population. Breastfeed. Med. 2019, 14, 465–474.
- Bahl, R.; Frost, C.; Kirkwood, B.R.; Edmond, K.; Martines, J.; Bhandari, N.; Arthur, P. Infant feeding patterns and risks of death and hospitalization in the first half of infancy: Multicentre cohort study. Bull. World Health Organ. 2005, 83, 418–426.
- Vennemann, M.M.; Bajanowski, T.; Brinkmann, B.; Jorch, G.; Yücesan, K.; Sauerland, C.; Mitchell, E.A. Does breastfeeding reduce the risk of sudden infant death syndrome? Pediatrics 2009, 123, e406–e410.
- Wisnieski, L.; Kerver, J.; Holzman, C.; Todem, D.; Margerison-Zilko, C. Breastfeeding and Risk of Metabolic Syndrome in Children and Adolescents: A Systematic Review. J. Hum. Lact. Off. J. Int. Lact. Consult. Assoc. 2018, 34, 515–525.
- Oddy, W.H. Breastfeeding, Childhood Asthma, and Allergic Disease. Ann. Nutr. Metab. 2017, 70 (Suppl. S2), 26–36.
- Martin, R.M.; Smith, G.D.; Mangtani, P.; Frankel, S.; Gunnell, D. Association between breast feeding and growth: The Boyd-Orr cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 87, F193.
- Mohammad, M.A.; Haymond, M.W. The magic of mother’s milk. Diabetes 2012, 61, 3076–3077.
- Rich-Edwards, J.W.; Stampfer, M.J.; Manson, J.E.; Rosner, B.; Hu, F.B.; Michels, K.B.; Willett, W.C. Breastfeeding during Infancy and the Risk of Cardiovascular Disease in Adulthood. Epidemiology 2004, 15, 550–556.
- Su, Q.; Sun, X.; Zhu, L.; Yan, Q.; Zheng, P.; Mao, Y.; Ye, D. Breastfeeding and the risk of childhood cancer: A systematic review and dose-response meta-analysis. BMC Med. 2021, 19, 90.
- Amitay, E.L.; Keinan-Boker, L. Breastfeeding and Childhood Leukemia Incidence: A Meta-analysis and Systematic Review. JAMA Pediatr. 2015, 169, e151025.
- Meier, P.P.; Patel, A.L.; Bigger, H.R.; Rossman, B.; Engstrom, J.L. Supporting breastfeeding in the neonatal intensive care unit: Rush Mother’s Milk Club as a case study of evidence-based care. Pediatr. Clin. N. Am. 2013, 60, 209–226.
- Intestinal Permeability in Preterm Infants by Feeding Type: Mother’s Milk Versus Formula. Breastfeed. Med. 2009, 4, 11–15.
- Camacho-Morales, A.; Caba, M.; García-Juárez, M.; Caba-Flores, M.D.; Viveros-Contreras, R.; Martínez-Valenzuela, C. Breastfeeding Contributes to Physiological Immune Programming in the Newborn. Front. Pediatr. 2021, 9, 3.
- Dimitroglou, M.; Iliodromiti, Z.; Christou, E.; Volaki, P.; Petropoulou, C.; Sokou, R.; Boutsikou, T.; Iacovidou, N. Human Breast Milk: The Key Role in the Maturation of Immune, Gastrointestinal and Central Nervous Systems: A Narrative Review. Diagnostics 2022, 12, 2208.
- Jølving, L.R.; Nielsen, J.; Kesmodel, U.S.; Nielsen, R.G.; Beck-Nielsen, S.S.; Nørgård, B.M. Prevalence of maternal chronic diseases during pregnancy—A nationwide population based study from 1989 to 2013. Acta Obstet. Et Gynecol. Scand. 2016, 95, 1295–1304.
- Scime, N.V.; Lee, S.; Jain, M.; Metcalfe, A.; Chaput, K.H. A Scoping Review of Breastfeeding in Women with Chronic Diseases. Breastfeed. Med. 2021, 16, 851–862.
- Colstrup, M.; Mathiesen, E.R.; Damm, P.; Jensen, D.M.; Ringholm, L. Pregnancy in women with type 1 diabetes: Have the goals of St. Vincent declaration been met concerning foetal and neonatal complications? J. Matern.-Fetal Neonatal Med. 2013, 26, 1682–1686.
- Lekšić, G.; Baretić, M.; Ivanišević, M.; Jurišić-Eržen, D. Pregnancy in Patients with Type One Diabetes Mellitus Treated with Continuous Subcutaneous Insulin Infusion-Preconception Basal Insulin Dose as a Potential Risk Factor for Fetal Overgrowth? Int. J. Environ. Res. Public Health 2020, 17, 6566.
- Smyth, A.; Oliveira, G.H.; Lahr, B.D.; Bailey, K.R.; Norby, S.M.; Garovic, V.D. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin. J. Am. Soc. Nephrol. CJASN 2010, 5, 2060–2068.
- Metcalfe, A.; Sabr, Y.; Hutcheon, J.A.; Donovan, L.; Lyons, J.; Burrows, J.; Joseph, K.S. Trends in Obstetric Intervention and Pregnancy Outcomes of Canadian Women With Diabetes in Pregnancy From 2004 to 2015. J. Endocr. Soc. 2017, 1, 1540–1549.
- Bramham, K.; Parnell, B.; Nelson-Piercy, C.; Seed, P.T.; Poston, L.; Chappell, L.C. Chronic hypertension and pregnancy outcomes: Systematic review and meta-analysis. BMJ (Clin. Res. Ed.) 2014, 348, g2301.
- Borba, V.V.; Zandman-Goddard, G.; Shoenfeld, Y. Prolactin and Autoimmunity. Front. Immunol. 2018, 9, 73.
- Buskila, D.; Shoenfeld, Y. Prolactin, bromocriptine and autoimmune diseases. Isr. J. Med. Sci. 1996, 32, 23–27.
- Fojtikova, M.; Tomasová Studýnková, J.; Filkova, M.; Lacinova, Z.; Gatterova, J.; Pavelka, K.; Vencovský, J.; Šenolt, L. Elevated prolactin levels in patients with rheumatoid arthritis: Association with disease activity and structural damage. Clin. Exp. Rheumatol. Incl Suppl. 2010, 28, 849.
- Song, G.G.; Lee, Y.H. Circulating prolactin level in systemic lupus erythematosus and its correlation with disease activity: A meta-analysis. Lupus 2017, 26, 1260–1268.
- Hilfiker-Kleiner, D.; Kaminski, K.; Podewski, E.; Bonda, T.; Schaefer, A.; Sliwa, K.; Forster, O.; Quint, A.; Landmesser, U.; Doerries, C.; et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007, 128, 589–600.
- Hilfiker-Kleiner, D.; Haghikia, A.; Berliner, D.; Vogel-Claussen, J.; Schwab, J.; Franke, A.; Schwarzkopf, M.; Ehlermann, P.; Pfister, R.; Michels, G.; et al. Bromocriptine for the treatment of peripartum cardiomyopathy: A multicentre randomized study. Eur. Heart J. 2017, 38, 2671–2679.
- Anaya, J.-M.; Shoenfeld, Y. Multiple autoimmune disease in a patient with Hyperprolactinemia. Isr. Med. Assoc. J. IMAJ 2005, 7, 740–741.
- Saha, S.; Gonzalez, J.; Rosenfeld, G.; Keiser, H.; Peeva, E. Prolactin alters the mechanisms of B cell tolerance induction. Arthritis Rheum. 2009, 60, 1743–1752.
- Leaños-Miranda, A.; Cárdenas-Mondragón, G. Serum free prolactin concentrations in patients with systemic lupus erythematosus are associated with lupus activity. Rheumatology 2006, 45, 97–101.
- Qian, Q.; Liuqin, L.; Hao, L.; Shiwen, Y.; Zhongping, Z.; Dongying, C.; Fan, L.; Hanshi, X.; Xiuyan, Y.; Yujin, Y. The effects of bromocriptine on preventing postpartum flare in systemic lupus erythematosus patients from South China. J. Immunol. Res. 2015, 2015, 316965.
- Praprotnik, S.; Agmon-Levin, N.; Porat-Katz, B.S.; Blank, M.; Meroni, P.L.; Cervera, R.; Miesbach, W.; Stojanovich, L.; Szyper-Kravitz, M.; Rozman, B.; et al. Prolactin’s role in the pathogenesis of the antiphospholipid syndrome. Lupus 2010, 19, 1515–1519.
- Orbach, H.; Zandman-Goddard, G.; Boaz, M.; Agmon-Levin, N.; Amital, H.; Szekanecz, Z.; Szucs, G.; Rovensky, J.; Kiss, E.; Doria, A.; et al. Prolactin and autoimmunity: Hyperprolactinemia correlates with serositis and anemia in SLE patients. Clin. Rev. Allergy Immunol. 2012, 42, 189–198.
- Bundhun, P.K.; Soogund, M.Z.; Huang, F. Impact of systemic lupus erythematosus on maternal and fetal outcomes following pregnancy: A meta-analysis of studies published between years 2001–2016. J. Autoimmun. 2017, 79, 17–27.
- Chen, Y.J.; Chang, J.C.; Lai, E.L.; Liao, T.L.; Chen, H.H.; Hung, W.T.; Hsieh, T.Y.; Huang, W.N.; Chen, Y.H.; Lin, C.H.; et al. Maternal and perinatal outcomes of pregnancies in systemic lupus erythematosus: A nationwide population-based study. Semin. Arthritis Rheum. 2020, 50, 451–457.
- Orefice, V.; Ceccarelli, F.; Pirone, C.; Galoppi, P.; Spinelli, F.R.; Alessandri, C.; Brunelli, R.; Perrone, G.; Conti, F. Breastfeeding in women affected by systemic lupus erythematosus: Rate, duration and associated factors. Lupus 2021, 30, 913–920.
- Eudy, A.M.; Siega-Riz, A.M.; Engel, S.M.; Franceschini, N.; Howard, A.G.; Clowse, M.E.B.; Petri, M. Effect of pregnancy on disease flares in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2018, 77, 855–860.
- Izmirly, P.M.; Costedoat-Chalumeau, N.; Pisoni, C.N.; Khamashta, M.A.; Kim, M.Y.; Saxena, A.; Friedman, D.; Llanos, C.; Piette, J.C.; Buyon, J.P. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation 2012, 126, 76–82.
- Leroux, M.; Desveaux, C.; Parcevaux, M.; Julliac, B.; Gouyon, J.B.; Dallay, D.; Pellegrin, J.L.; Boukerrou, M.; Blanco, P.; Lazaro, E. Impact of hydroxychloroquine on preterm delivery and intrauterine growth restriction in pregnant women with systemic lupus erythematosus: A descriptive cohort study. Lupus 2015, 24, 1384–1391.
- Guillotin, V.; Bouhet, A.; Barnetche, T.; Richez, C.; Truchetet, M.E.; Seneschal, J.; Duffau, P.; Lazaro, E. Hydroxychloroquine for the prevention of fetal growth restriction and prematurity in lupus pregnancy: A systematic review and meta-analysis. Jt. Bone Spine 2018, 85, 663–668.
- Noviani, M.; Wasserman, S.; Clowse, M.E. Breastfeeding in mothers with systemic lupus erythematosus. Lupus 2016, 25, 973–979.
- Acevedo, M.; Pretini, J.; Micelli, M.; Sequeira, G.; Kerzberg, E. Breastfeeding initiation, duration, and reasons for weaning in patients with systemic lupus erythematosus. Rheumatol. Int. 2017, 37, 1183–1186.
- Al-Herz, A.; Schulzer, M.; Esdaile, J.M. Survey of antimalarial use in lupus pregnancy and lactation. J. Rheumatol. 2002, 29, 700–706.
- American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics 2001, 108, 776–789.
- Megan, E.B.C.; Amanda, M.E.; Stephen, B.; Gillian, S.-S.; Andrzej, K.; Rebecca, F.-B.; Dafna, D.G.; Yair, M.; Cecilia, N.; Abir, M.; et al. Hydroxychloroquine in the pregnancies of women with lupus: A meta-analysis of individual participant data. Lupus Sci. Med. 2022, 9, e000651.
- Clapp, C.; Ortiz, G.; García-Rodrigo, J.F.; Ledesma-Colunga, M.G.; Martínez-Díaz, O.F.; Adán, N.; Martínez de la Escalera, G. Dual Roles of Prolactin and Vasoinhibin in Inflammatory Arthritis. Front. Endocrinol. 2022, 13, 905756.
- Silman, A.; Kay, A.; Brennan, P. Timing of pregnancy in relation to the onset of rheumatoid arthritis. Arthritis Rheum. 1992, 35, 152–155.
- Brennan, P.; Silman, A. Breast-feeding and the onset of rheumatoid arthritis. Arthritis Rheum. 1994, 37, 808–813.
- Barrett, J.H.; Brennan, P.; Fiddler, M.; Silman, A. Breast-feeding and postpartum relapse in women with rheumatoid and inflammatory arthritis. Arthritis Rheum. 2000, 43, 1010–1015.
- Eudy, A.M.; McDaniel, G.; Clowse, M.E.B. Pregnancy in rheumatoid arthritis: A retrospective study. Clin. Rheumatol. 2018, 37, 789–794.
- Ince-Askan, H.; Hazes, J.M.W.; Dolhain, R. Breastfeeding among Women with Rheumatoid Arthritis Compared with the General Population: Results from a Nationwide Prospective Cohort Study. J. Rheumatol. 2019, 46, 1067–1074.
- Nielsen, O.H.; Andreasson, B.; Bondesen, S.; Jacobsen, O.; Jarnum, S. Pregnancy in Crohn’s disease. Scand. J. Gastroenterol. 1984, 19, 724–732.
- Beaulieu, D.B.; Kane, S. Inflammatory bowel disease in pregnancy. World J. Gastroenterol. 2011, 17, 2696–2701.
- Kane, S.; Kisiel, J.; Shih, L.; Hanauer, S. HLA disparity determines disease activity through pregnancy in women with inflammatory bowel disease. Am. J. Gastroenterol. 2004, 99, 1523–1526.
- Mahadevan, U.; Sandborn, W.J.; Li, D.K.; Hakimian, S.; Kane, S.; Corley, D.A. Pregnancy outcomes in women with inflammatory bowel disease: A large community-based study from Northern California. Gastroenterology 2007, 133, 1106–1112.
- Dotan, I.; Alper, A.; Rachmilewitz, D.; Israeli, E.; Odes, S.; Chermesh, I.; Naftali, T.; Fraser, G.; Shitrit, A.B.-G.; Peles, V.; et al. Maternal inflammatory bowel disease has short and long-term effects on the health of their offspring: A multicenter study in Israel. J. Crohn’s Colitis 2013, 7, 542–550.
- Moffatt, D.C.; Ilnyckyj, A.; Bernstein, C.N. A Population-Based Study of Breastfeeding in Inflammatory Bowel Disease: Initiation, Duration, and Effect on Disease in the Postpartum Period. Off. J. Am. Coll. Gastroenterol. ACG 2009, 104, 2517–2523.
- Kane, S.; Lemieux, N. The Role of Breastfeeding in Postpartum Disease Activity in Women with Inflammatory Bowel Disease. Off. J. Am. Coll. Gastroenterol. ACG 2005, 100, 102–105.
- Orholm, M.; Fonager, K.; Sørensen, H.T. Risk of ulcerative colitis and Crohn’s disease among offspring of patients with chronic inflammatory bowel disease. Am. J. Gastroenterol. 1999, 94, 3236–3238.
- Koletzko, S.; Sherman, P.; Corey, M.; Griffiths, A.; Smith, C. Role of infant feeding practices in development of Crohn’s disease in childhood. BMJ: Br. Med. J. 1989, 298, 1617.
- Bove, R.; Chitnis, T. The role of gender and sex hormones in determining the onset and outcome of multiple sclerosis. Mult. Scler. 2014, 20, 520–526.
- Bove, R.; Alwan, S.; Friedman, J.M.; Hellwig, K.; Houtchens, M.; Koren, G.; Lu, E.; McElrath, T.F.; Smyth, P.; Tremlett, H.; et al. Management of multiple sclerosis during pregnancy and the reproductive years: A systematic review. Obstet. Gynecol. 2014, 124, 1157–1168.
- Wundes, A.; Pebdani, R.N.; Amtmann, D. What do healthcare providers advise women with multiple sclerosis regarding pregnancy? Mult. Scler. Int. 2014, 2014, 819216.
- Krysko, K.M.; Rutatangwa, A.; Graves, J.; Lazar, A.; Waubant, E. Association Between Breastfeeding and Postpartum Multiple Sclerosis Relapses: A Systematic Review and Meta-analysis. JAMA Neurol. 2020, 77, 327–338.
- Vukusic, S.; Hutchinson, M.; Hours, M.; Moreau, T.; Cortinovis-Tourniaire, P.; Adeleine, P.; Confavreux, C. Pregnancy and multiple sclerosis (the PRIMS study): Clinical predictors of post-partum relapse. Brain 2004, 127, 1353–1360.
- Portaccio, E.; Ghezzi, A.; Hakiki, B.; Sturchio, A.; Martinelli, V.; Moiola, L.; Patti, F.; Mancardi, G.L.; Solaro, C.; Tola, M.R.; et al. Postpartum relapses increase the risk of disability progression in multiple sclerosis: The role of disease modifying drugs. J. Neurol. Neurosurg. Psychiatry 2014, 85, 845–850.
- Jesus-Ribeiro, J.; Correia, I.; Martins, A.I.; Fonseca, M.; Marques, I.; Batista, S.; Nunes, C.; Macário, C.; Almeida, M.C.; Sousa, L. Pregnancy in Multiple Sclerosis: A Portuguese cohort study. Mult. Scler. Relat. Disord. 2017, 17, 63–68.
- Hellwig, K.; Rockhoff, M.; Herbstritt, S.; Borisow, N.; Haghikia, A.; Elias-Hamp, B.; Menck, S.; Gold, R.; Langer-Gould, A. Exclusive Breastfeeding and the Effect on Postpartum Multiple Sclerosis Relapses. JAMA Neurol. 2015, 72, 1132–1138.
- Langer-Gould, A.; Smith, J.; Albers, K.; Wu, J.; Kerezsi, E.; McClearnen, K.; Leimpeter, A.; Van Den Eeden, S. Pregnancy-related relapses and breastfeeding in a contemporary multiple sclerosis cohort. Neurology 2019, 94, e1939–e1949.
- Pakpoor, J.; Disanto, G.; Lacey, M.V.; Hellwig, K.; Giovannoni, G.; Ramagopalan, S.V. Breastfeeding and multiple sclerosis relapses: A meta-analysis. J. Neurol. 2012, 259, 2246–2248.
This entry is offline, you can click here to edit this entry!
