Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
The orexinergic system is involved in the control of the sleep/waking cycle, and it has been reported that cancer and cancer-related inflammatory mechanisms are associated with fatigue and sleep disruption. A bidirectional relationship between cancer and sleep occurs, and circadian rhythm disorders are a risk factor for some cancers. Thus, drugs targeting orexin receptors and administered for the treatment of sleep disorders could also be used against certain tumors
- orexin A
- orexin B
- orexin receptor
1. Precursor and Peptides
The orexinergic system includes orexins A and B and the orexin receptors type 1 (OXR1) and 2 (OXR2) [14], and integrated with other systems, such as leptin and ghrelin systems, controls the energy homeostasis [13], and is essential for the normal consolidation of sleep and waking [18]. Orexin A and orexin B (also known as hypocretin 1 and 2, respectively) are encoded by the same gene (hcrt) on chromosome 17 [1] and are constituted of two exons [16] being the enzymatic cleavage products of a single 130-residue precursor, prepro-orexin, and share a 46% amino acid identity [1,19,20]. Both peptides have an amidated C-terminal end and include two α-helices domains linked by a small flexible domain [15,21]. Orexin A includes residues 28–66 of the prepro-orexin (33-residue peptide) and shows two intramolecular disulfide bridges within the N-terminal that are not essential for its full activity [22]. The C-terminal domain (sequence 20 to 33) is essential for its activity, and the deletion of the central domain between residues 15 and 19 strongly reduced the peptide activity [22]. Orexin B is a linear 28-residue peptide, corresponding to prepro-orexin residues 69–97 [1,19,20], without disulfide bridges [1]. The C-terminal domain of orexin B is crucial for its activity, but the deletion of the first six residues of the peptide have no impact on it [22,23]. It has been reported that some single-nucleotide polymorphisms corresponding to missense mutation, mainly located in the C-terminal domain of both peptides, may have a negative impact on the peptide activity [24].
In an adult brain, in situ hybridization displayed, via cRNA probe, neurons containing prepro-orexin distributed in hypothalamic (lateral and posterior hypothalamic areas and perifornical nucleus) and subthalamic (zona incerta, subincertal, and subthalamic nuclei) areas [25,26]. In addition, neurons containing the peptides were detected by immunohistochemistry in the dorsal lateral hypothalamic area, and immunoreactive fibers were observed in the arcuate hypothalamic nucleus [25]. These neurons display extensive and wide projections to numerous regions of the central nervous system [25,26], and thus, orexins can regulate many physiological functions, although the heaviest projections are related to regions that control arousal and wakefulness [13]. The hypothalamic neurons producing orexins can be segregated into distinct populations (anatomically, genetically, and functionally) which are involved in the different functions regulated by orexins, such as reward, stress, emotions, feeding, sleep/wakefulness cycle, or arousal [27]. In addition, orexin has also been detected in the enteric nervous system and in the enteroendocrine gut cells [28], as well as in the reproductive tract, pituitary, adipocytes, thyroid, pancreas, adrenal gland, testis, and ovary [29].
2. Receptors
Orexins A and B exert their functions throughout receptors type 1 (OXR1) and 2 (OXR2), which are G-protein-coupled receptors with seven transmembrane domains [13,18,26] belonging to the large class A rhodopsin-like subfamily of G-protein-coupled receptors [30]. OXR1 is encoded by the hcrtr1 gene located on chromosome 1, and OXR2 is encoded by the hcrtr2 gene located on chromosome 6 [25,26]. The two receptors shared 64% of identity sequence [31] and can bind orexin A with the same affinity, and orexin B shows a better affinity for OXR2 than for OXR1 [3]. The identification of OXR2, but not OXR1, in non-mammals suggests that OXR2 could be the ancestral receptor form [21,32].
The two orexin receptors lead to signal transduction by activation and/or inhibition of different intracellular signaling pathways to obtain final cellular responses [33]. The binding of ligands to their respective receptors induces a structural conformational change and the activation of G proteins [34]. At least three G-protein families can couple to both orexin receptors: Gq, Gi/o, and Gs [18]. Like other receptors coupled to G proteins, orexin receptors (OXRs) may be engaged in dimerization/oligomerization processes, leading to homomeric and heteromeric complexes; these latter might affect receptor trafficking, signaling, or pharmacology [35,36]. For example, heterodimers between human OXR1 and the CB1 cannabinoid receptor have been verified, but the physiological significance of these interactions has not been completely dilucidated [18].
OXRs did not show significant affinity for other peptides. The transmembrane domains 1, 3, and 5 and the amino terminus of the receptors account for the interaction with the orexin peptides, and the transmembrane domain 3 is critical for receptor interactions with small molecule antagonists [37]. OXRs and the families of G proteins coupled with them regulate non-selective cation channels, phospholipases, and adenylyl cyclase, as well as protein and lipid kinases, and plastic effects are observed in some cell types [18]. Both OXRs exhibit slow kinetics in their response to orexin binding [37] and are widely distributed throughout the central nervous system but show a more restricted expression in the peripheral nervous system. Although a certain degree of overlap has been observed, the two receptors display different distributions [13]. It is well known that OXRs regulate homeostatic processes [38,39,40,41,42]. The most significant effect of orexin upon cells is the depolarization of neurons leading to increasing excitability and firing rate [43,44,45,46], and this depolarization is produced by the activation of a non-selective cation current [18]. Although the mechanisms underlying OXR signaling in the central nervous system are mostly unknown, orexin responses in neurons include the modulation of presynaptic transmitter release and changes in synaptic plasticity [47,48,49,50]. In experimental animals it has been described that the absence of one or both OXRs affects the markers of cholinergic transmission [51,52]. In addition, the participation of the orexigenic system in human diseases (e.g., narcolepsy, obesity, drug addiction) has been reported [38,39,40,41].
It has been reported that the regulatory phosphorylation site (Ser-262) on the OXR1 did not affect its interaction with beta-arrestin1/2, G proteins or GRK (G-protein-coupled receptor kinase) 5 but abolished its interaction with GRK2 [53]. This means that Ser-262 is involved in the internalization of OXR1 and in promoting a GRK2-dependent biased signaling through orexin A. Moreover, the biased signaling between beta-arrestin/G protein is a promising strategy to improve drug efficacy [53]. It has been suggested that OXR1-selective antagonists might be potential anti-addiction drugs, but the role of OXR2 in drug seeking has not been established. The blockade of this latter receptor as pharmacological treatment for addiction should be considered cautiously because of the predominant physiological consequence of OXR2 in wakefulness regulation [13]. The determination of the structures of both OXRs allows for analyzing interactions between receptors and antagonists, with the aim of designing new antagonist molecules [54].
The activation of OXRs may also produce long-term plastic effects. For example, it has been reported that placentally produced orexins may contribute to the prenatal development of brown adipose tissue in mice via OXR1 [50,55]. It has been reported that orexin A and OXR1 are present in low amounts in blood, and they do not follow a circadian pattern [42]. The serum orexin A level was lower in normal patients than that found in patients with cancer cachexia [56], and a lower level of this peptide was accompanied by elevated expression of OXR2 in benign prostatic hyperplasia [57]. In addition, the epigenetic silencing of OXR2 was in association with endometrial cancer [58]. A decrease in food consumption and body weight is frequently observed in patients with cancer cachexia, as well as anorexia and fatigue [59]: the Japanese herbal medicine Ninjinyoeito (NYT, contains twelve herbal crude drugs) is prescribed to attenuate these symptoms, since it promotes hyperphagia. It has been reported that one of the tested NYT formulations without Citrus unshiu peel failed to activate OXR1; by contrast, Citrus unshiu peel activated this receptor, which was blocked with SB-674,042, a selective OXR1 antagonist [59]. Thus, Citrus unshiu peel, after activating the hypothalamic neurons expressing OXR1, augmented food intake; this suggests the potential use of NYT in cancer patients with anorexia. In addition, an abnormal expression of OXR1 was observed in human peripheral organs in pathologic conditions [14]; for instance, primary colorectal tumors express OXR1 [60]. This observation emphasizes the potential therapeutic importance of OXRs. More specifically, OXR activation has been suggested to be a promising treatment for chemotherapy-resistant carcinoma [14,60].
3. Signaling Pathways
The two major signal transduction pathways associated with ligand–receptor binding are the cAMP pathway (through the adenylyl cyclase effector) and the phosphatidylinositol signal pathway (through the phospholipase C effector) [61]. OXR signaling is highly diverse depending on the milieu in which they are operating, and different responses have been observed at different sites [18,62]. Orexins, via OXRs, activate phospholipase A2, C and D, diacylglycerol lipase, PI3K (phosphatidylinositol 3-kinase)-Akt (protein kinase B), adenylyl cyclase/cAMP, and MAPK (mitogen-activated protein kinases)-ERK (extracellular-signal-regulated kinases) 1/2 and JNK (jun amino-terminal kinases) signaling cascades [18,63]. The two OXRs may couple to several G-protein species; thus, although the Gq phospholipase C (PLC) pathway plays an important role in many cases [62], the idea that OXR1 couples only to Gq and OXR2 couples to Gq and Gi/o needs to be reconsidered [18], and no evidence shows significantly different signaling of the two OXR subtypes [62]. In general, OXRs coupled with Gq proteins stimulate intracellular calcium via PLC [13,18,62]. They are also capable of regulating adenylyl cyclase, but this action seems less prominent than coupling to the PLC and calcium cascades [18].
In general, changes in the structure of the receptor after ligands binding [37] trigger a protein kinase C (PKC)-mediated influx of calcium across the plasma membrane via L-type calcium channels [13]. The activation of the calcium channels is related to many other signaling pathways, including the activation of MAPK, especially the ERK p38, cAMP-response element binding protein (CREB), adenylyl cyclase, and PLC [13]. The main responses of activation of OXRs in neurons are the inhibition of K+ channels and the activation of the Na+/Ca2+ exchanger and non-selective cation channels of unknown identity, although the G protein implicated as the main signal transducer in these cells is still unknown [62]. Both OXRs strongly activate PLC [18], and intracellular calcium stores are released from the endoplasmic reticulum by a PLC-mediated pathway, producing sustained excitation of related neurons [64,65,66,67] and inducing cell responses mainly via Ca2+- and diacylglycerol-mediated pathways [18]. Orexin B induced ERK1/2 activation through OXR2, predominantly through the Gq/PLC/PKC pathway [68], although this receptor can differentially couple to Gq, Gi/o, and Gs proteins in different tissues [69,70]. Anyway, PLC activation may be a very central OXR cascade, and the role of PLC might be to produce Ca2+ elevation or to elevate diacylglycerol for possible PKC activation [18]. Under certain circumstances, the Gq-PLC pathway may take the route to diacylglycerol lipase, leading to the production of the endocannabinoid 2-arachidonoyl glycerol and connecting orexins with endocannabinoid signaling [62]. This interaction between orexins and endocannabinoids has been reported, with indirect evidence, for neuronal circuits regulating antinociception and reward seeking, as well as an autocrine mechanism in orexigenic neurons [71]. Orexin activates reward pathways by upregulating the addiction-related neurotransmitters and receptor activity [13]. It has been reported that OXR1 signaling is a part of the dopamine-mediated reward circuit, and that the increased orexin signaling (application of orexin A or enhancement of OXR2) induced a glutamate-stimulated effect on alcohol consumption, an activation that promoted drug seeking via a PLC/PKC pathway [13]. Orexins increase the GH-releasing hormone that stimulated GH release through an L-type Ca2+ current and PKC-mediated signaling pathways [72,73]. In addition, other authors report that mechanisms involved in the central control of metabolism, such as AMPK, unfolded protein response, and endoplasmic reticulum stress, are related to the control of energy homeostasis by the orexinergic system [42].
Orexin signaling induces apoptosis in some cells, whereas in others, orexin signaling raises proliferative activity [13]. Persistent stimulation of OXRs induces programmed cell death [18]; moreover, the pro-apoptotic property of orexin signaling is highly cell type dependent, and this may be due to the pathways induced mainly by the activation of OXR1. Activation of this receptor drives apoptosis via the Gq protein but independent of classical Gαq activation of PLC [13]. The signal cascade was suggested to involve (in addition of Gq) Src or a related kinase, phosphorylation of OXR1 and the recruitment of the protein phosphatase SHP-2. It has been reported that p38 MAPK could be the carrier of the cell death response [18], and the neuroprotective actions of orexin A might be mediated by the PKC and PI3K signaling pathways [34]. On the other hand, the activation of OXR1 promotes cell proliferation in some cancer cell types (pancreatic PANC1 cancer cells) through inhibiting cell apoptosis. It has been reported that the Akt/mammalian target of rapamycin (mTOR) signaling pathway is involved in this mechanism, leading to cell proliferation by the inhibition of Bcl-2/caspase-9/c-myc-mediated apoptosis [42]. Figure 1 shows the main signaling pathways mediated by orexins.
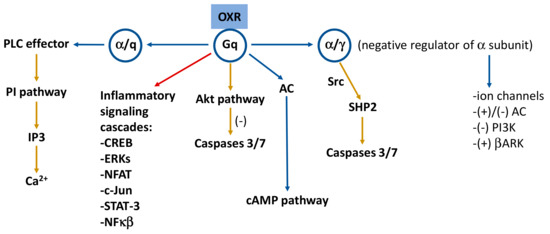
Figure 1. Signaling pathways mediated by the orexinergic system. Receptor subunits are included in circles. Yellow arrows indicate neuroprotective or anti-inflammatory pathways and red arrow points to inflammatory signaling cascades. Based on references [1,15,34]. AC: adenylate cyclase; Akt: protein kinase B; βARK: G protein-coupled receptor kinases; cAMP: cyclic adenosine monophosphate; CREB: cAMP-response-element-binding protein; ERKs: extracellular signal-regulated kinases; IP3: inositol-1,4,5-trisphosphate; NFAT: nuclear factor of activated T cells; NFκB: nuclear factor kappa light chain enhancer of activated B cells; PI: phosphoinositide; PI3K: phosphoinositide 3-kinase; PLC: phospholipase C; SHP2: Src homology 2 (SH2) domains of SH2-containing phosphatase 2; Src: Src (non-receptor tyrosine) kinases; STAT-3: signal transducer and activator of transcription 3; (+): activation; (−): inhibition.
4. Physiological and Pathophysiological Actions
The major biological action of orexins is the regulation of sleep/wakefulness state [74] but they are also involved in drug addiction, motivation, food consumption, homeostasis, hormone secretion, reproductive function, lipolysis, and blood pressure regulation [1,15]. In particular, orexinergic neurons perceive rapidly the body’s nutritional status and respond to metabolic signals; for instance, the hypothalamic expression of the orexin precursor is increased during fasting, and as a part of the survival strategy elevated orexin levels have been detected during food deprivation periods [75,76,77,78]. Moreover, the orexinergic system exerts neuroprotective, immunoregulatory and anti-inflammatory effects and has been involved in high fat diet-induced obesity, intestinal bowel diseases, neuroinflammation, multiple sclerosis, Alzheimer’s disease, and septic shock [34]. Orexins are also related to pathologies such as narcolepsy, metabolic syndrome, and cancer [15]. In this sense, the orexinergic system can trigger opposite mechanisms depending on the environment. Thus, it seems that the cellular signaling after the activation of OXRs is more complex than that of other receptors.
The orexigenic system is very sensitive to the action of certain drugs and is closely related to addiction-reward processes because it inhibits the activity of the insula, a brain region involved in these functions by acting on the reward circuit [13]. In addition, the role of orexins on the regulation of central cardiovascular mechanisms has been demonstrated since the action of orexin A on some cardiovascular centers of the brainstem leads to the inhibition of vagal activity and the increase of sympathetic activity to the heart, as well as the alteration of the excitability of central cardiovascular circuits [13]. It has been proposed that the actions of orexins on the regulation of blood pressure and heart rate could be mediated throughout α- or β-adrenoreceptors [13].
The orexinergic system also regulates endocrine axes [13] and may operate as putative neuroendocrine and autocrine/paracrine regulators of gonadal function [79]. The detection of OXRs in peripheral tissues suggests a paracrine action of orexins; in addition, the circulating levels of orexins in blood in healthy individuals is very low [34]. The abnormal expression of OXRs in some human pathologies may lead to new therapeutic approaches, and it has been suggested that orexins or the orexin neural circuitry represent potential targets for the treatment of multiple pathologies related to inflammation including multiple sclerosis, obesity, septic shock, or several types of cancer [1,14].
This entry is adapted from the peer-reviewed paper 10.3390/app13137596
This entry is offline, you can click here to edit this entry!
