Gait analysis can be performed through laboratory systems, non-wearable sensors (NWS), and/or wearable sensors (WS). Using these tools, physiotherapists and neurologists have more objective measures of motion function and can plan tailored and specific gait and balance training early to achieve better outcomes and improve patients’ quality of life.
- gait analysis
- neurorehabilitation
- neurological disorders
- wearable sensors
- non-wearable sensors
1. Introduction
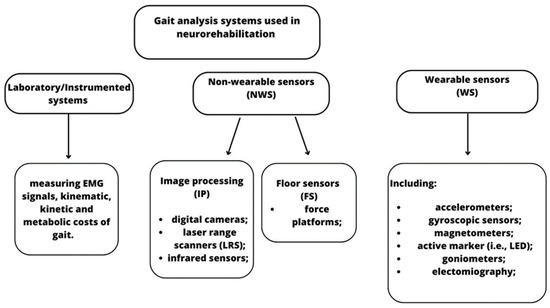
2. Neurodegenerative Disorders
|
Reference n° |
Gait Analysis System |
Technology Description |
Neurological Disorder |
Clinical Implication |
|
|---|---|---|---|---|---|
|
Non- Wearable Sensors |
Wearable Sensors |
||||
|
[15] |
X |
X |
Three-dimensional gait analysis in laboratory, including optometric system, a dynamometric platform, and ad hoc software. |
PD with 1.5–2 H&Y stage |
Reduced gait speed and step length, showing bilateral extra rotation of knee, ankle, and foot. |
|
[16] |
X |
Triaxial accelerometer-based device placed on the fifth lumbar vertebrae and a double-sided tape. |
PD with 1–3 H&Y stage |
NA |
|
|
[17] |
X |
Instrumented force-sensitive insole placed in patients’ shoes, with eight pressure-sensitive sensors. |
PD with 2–3 H&Y stage |
Stride-to-stride variability due to bradykinesia, loss of muscle synergies in the lower limb, and lack of rhythmicity. |
|
|
[18] |
X |
X |
Motion-capture based gait analysis compared to mobile sensor (inertial sensors) gait analysis, which were integrated in the mid-sole of the athletic shoes. |
PD with 1–4 H&Y stage |
Reduced gait speed, stride time, and length; increased duration stance phase time accompanied by a synchronic decreasing duration of swing phase time. |
|
[19] |
X |
X |
Gait assessment through an optoelectronic (48 retroflected markers), inertial, and a smartphone-based capture system. |
PD with <3 H&Y stage |
NA |
|
[20] |
X |
Wearable device compared to Opti Track system, using an error state Kalman filter algorithm. |
PD |
NA |
|
|
[21] |
X |
Stereophotogrammetric system (Vicon Motion Systems Ltd., Oxford, UK) and reflective markers to estimate joints’ angles. |
MS with a score of ≤5–6 |
MS patients showed reduced gait speed, which correlated with a decrease in cadence, step length, and swing time, and an increase in stance time. Additionally, authors found an increased pelvic tilt, which negatively correlates with the 6MWT. |
|
|
[22] |
X |
X |
Wireless AS200 system, comprising three line-scanning camera system and 11 active infrared markers attached on body’s patient, with a 2-mm accuracy. |
MS with a mean score of 3.6 in EDSS |
MS patients manifested changes in variability of movement gait patterns due to fatigue, altered motor coordination linked to additional activity of the antagonists, or insufficient strength produced by the agonists. |
|
[23] |
X |
Walkway sensor and machine learning (XGB) process to distinguish MS patients’ degree of severity based on their gait features. |
MS with a mean score of 2.11 in EDSS |
Step time and step width were considered as the most important variables to distinguish level of severity of MS subjects. |
|
|
[9] |
X |
X |
SMART-E stereophotogrammetric system (BTS, Milan, Italy) with eight infrared cameras (for acquiring kinematic data). Sensorized pathway with 2 piezoelectric force platforms (for acquiring kinetic data), 22 retro-reflective spherical markers for lower-body segments, and 15 markers for the upper body, placed on specific anatomic sites. |
Spino-CA autosomal dominant (type 1 and 2) and Friedreich’s ataxia as recessive ataxia |
Loss of lower limbs control during gait and of ability to stabilize a walking strategy over time. CA patients definitively lack a stable gait control behavior since the cerebellum functions of motor behavior and developing new motor patterns are altered. |
|
[24] |
X |
Triaxial accelerometer. |
Spino-CA with a mean score of 3.9 for stance and gait in SARA |
Gait velocity, cadence, step length, step regularity, and step repeatability are strongly correlated with disease duration. |
|
|
[25] |
X |
Seven inertial sensors while performing two independent trials of gait and balance assessments. |
CA |
NA |
|
|
[26] |
X |
Three Opal inertial sensors were attached on both feet and the posterior trunk at the level of L5 with elastic Velcro bands. |
Spino-CA with a mean score of 3.6 for stance and gait in SARA |
Minimal changes in gait spatial–temporal parameters can be considered as accurate markers for CA progression. |
|
3. Acquired Brain Injury
|
Reference n° |
Gait Analysis System |
Technology Description |
Neurological Disorder |
Clinical Implication |
|
|---|---|---|---|---|---|
|
NWS |
WS |
||||
|
[38] |
X |
A 10 m walkway with a pressure sensitive mat. Spatial–temporal parameters were registered using GaitRite mat, which contains a total of 13,824 sensors. |
Post-stroke patients (both ischemic and hemorrhagic) |
Most useful gait parameters are step length, swing time, and stance time. In addition, authors stated that asymmetry time values are not reliable parameters to assess gait in post-stroke patients. |
|
|
[39] |
X |
Inertial Measurment Unit (IMU) system (Xsens Technology B.V., Enschede, The Netherlands, Hengelo) composed of seven inertial sensors. |
Post-stroke patients |
NA |
|
|
[40] |
X |
Kinect v2, which included an 8-core Intel® in addition to an ad hoc application designed to register the 3D position and orientation of the 25 human joints provided by the Kinect v2. |
Post-stroke patients (both ischemic and hemorrhagic) |
Results indicated that patients with a higher fall risk manifested lower gait velocity and cadence, a shorter stride and step length, and higher double support time. Additionally, the risk of falling was related to increased trunk and pelvic obliquity and tilt, and to decreased hip flexion–extension and ankle height variation. |
|
|
[41] |
X |
Odonate 3D motion capture system in a mobile terminal and a workstation. This innovative a binocular depth camera combined with an artificial intelligence system to capture, analyze, and calculate gait parameters automatically. |
Post-stroke patients |
Alterations were found in spatial–temporal and kinematic parameters; thus, this new system can perform an objective gait assessment in five minutes, also in a home-based setting. |
|
|
[42] |
X |
Five synchronized IMUs. |
Severe TBI patients |
Severe TBI patients present serious difficulties in maintaining balance during gait, especially movements of the head, which are the most impaired, probably related to vestibular dysfunctions due to traumatic events. Additionally, authors suggested to assess gait through dynamic balance skills during curved trajectories as in Figure-of-8 Walk Test. |
|
|
[43] |
X |
Three IMUs were attached with elastic straps over both lateral ankles to detect gait phases and over the fifth lumbar vertebrae. |
TBI |
TBI patients manifest great imbalances in dynamic balance, especially in antero-medial weight shifting, when compared with healthy control subjects. |
|
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering10070785
