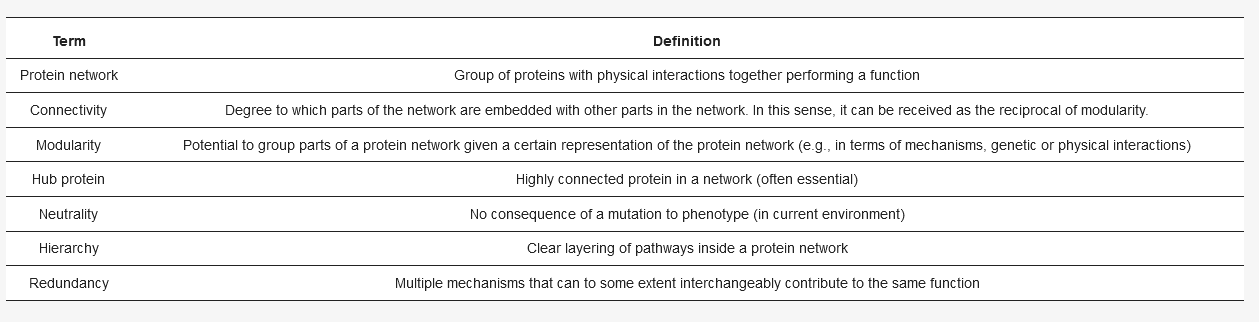
Every cell consists of many different interconnected functional protein networks (for definitions, see ), such as transcription, translation, or polarity establishment [
2]. The network’s architecture, (for example: which protein binds to/reacts with which other protein), impacts the evolutionary possibilities of a network in multiple ways. For example, hubs, proteins with many binding partners, tend to evolve slower [
3]. Less connected proteins, that may be deleted in a cell without a detectable change in cell physiology, can permit duplication of other genes and thus promote evolution [
4]: duplicates of a gene enable new options for diversification, which facilitate further evolution of a gene/protein and the surrounding network [
5,
6,
7]. Interestingly, many mutations (from 3% of non-silent mutations in bacteria to 30% in hominids [
8]) in a cell show very weak, or no effect on the cell’s function, a phenomenon called neutrality [
9]. Thus, proteins that may seem unimportant for how the cell works now, in this environment, may become important when changes occur in the network architecture due to a mutation or a switch in environment [
10,
11].
A well-studied model organism to concretize how these proteins, without a detectable phenotype, shape a network is
Saccharomyces cerevisiae, or budding yeast. The organism generally exhibits many of the network properties defined in , such as hierarchy and the presence of hubs [
2]. Here, neutrality is also pervasive, as only 40% of homozygous gene deletions for the entire organism initially had obvious phenotypes [
12]. Moreover, the environment has been shown to have a notable influence on neutrality, as lethal heterozygous deletions can be compensated by poor medium [
13]. As a general rule, both the network architecture and the environment can mask the function of many proteins.
Within this organism, a, to some extent, representative example of a protein network is the polarity network, which governs how the yeast chooses a direction in which to divide and involves directing dozens of proteins in a process of breaking its internal spherical symmetry (see, e.g., [
14]). As required, we observe the presence of the common network properties demonstrated in
Section 2, such as hierarchy and redundancy. Polarity is also one of many biological functions in yeast for which a subdivision of many proteins into a quasi-modular network proved possible [
15]. Within polarity, even more detailed submodules can be distinguished [
16]. Neutrality is also exhibited by several polarity proteins, which is in part responsible for the difficulty in determining the role of each protein. Lastly, polarity is a pattern formation process where, by definition, spatiotemporal dependencies are important, and understanding evolution generally relies on understanding this type of dependencies [
17].
However, the polarity network is not a prime example of the sort of networks with abundant transcriptional regulation. Other templates are better suited for learning about the evolution of gene regulation, such as interaction networks centered around transcriptional regulators (e.g., Mcm1 [
18]), ribosomal regulation (e.g., [
19]) the stress response (e.g., [
20]) or metabolic response (e.g.,
GAL pathway [
21]). The existence of established regulatory templates thus conveniently complements our focus on symmetry breaking during polarity as a model for the protein interaction network, which is also a topic for evolutionary studies.
Concretely, in [
22] a mutant strongly defective in polarity establishment was experimentally evolved and found to recover remarkably reproducibly, e.g., the first rescuing mutation to sweep the population was always the same. Because of this exhibited tractability of the adaptations, network structures within the polarity network that facilitated evolution could be concretely interpreted in terms of redundancies [
23]. In another approach to determine the flexibility of the polarity network, historical evolution was studied for 40+ proteins in almost 300 fungal species in [
11]. Again, the polarity network exhibited sufficient modularity so that studying its evolution separately from other functions still yielded interpretable results. For example, authors showed that polarity network size was shaped in part by fungal lifestyle (e.g., uni- or multicellular).
Nevertheless, clear justification for observed evolutionary trajectories in this network remain difficult to make. It is sometimes possible to generate more abstract predictions by linking network architecture to the evolvability of the polarity network using classical regulatory motifs. For example, the presence of positive and negative feedback in polarity establishment confers robustness to the network [
24,
25], which in turn may facilitate evolution. But in order to make more concrete and detailed predictions about evolutionary trajectories, an important insight is that we need mechanistic information of (parts of) the polarity network.
To illustrate this point, a bottom-up model was constructed in [
26] where molecular details were coarse-grained following analysis on numerical simulations of multiple polarity mechanisms [
23]. This approach was successful in quantitatively describing the fitness along the evolutionary trajectory in [
22]. Furthermore, the predictions on epistasis, an important bottleneck for predicting evolution [
27], can be extended to other modules, and although use of full mechanistic understanding is superior in quantitative assessments of epistasis, biofunctional information (viz. from GO-terms, in agreement with [
28]) as input to the model suffices for epistasis sign predictions.
Instructive is the Nrp1 case where full information is absent, but some phenomenological information is available. Based on the latter, inclusion of Nrp1 into the bottom-up coarse-grained model of [
26] is still worthwhile, but leaves room for improvement. This marks the importance of continuing to investigate protein networks until molecular mechanisms have been elucidated. In this review we advocate that obtaining this knowledge is (soon) feasible, motivating the use of the yeast polarity system for studies in network organization (with properties such as hierarchy, modularity/connectivity and redundancy that can “hide” proteins) as well as evolution.
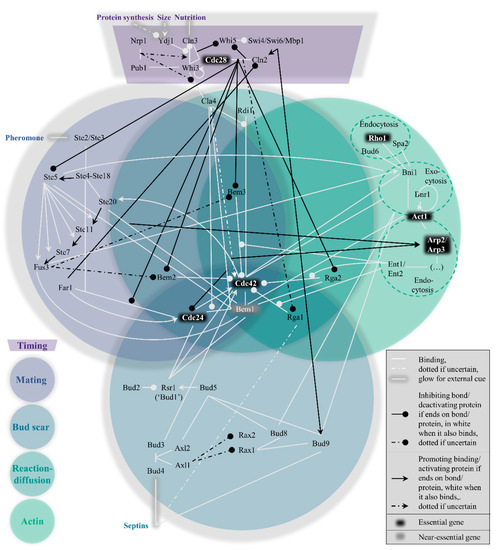
Within the yeast polarity network, four pathways to polarization exist which cannot easily be considered modular. Their interconnectivity can be conveniently visualized in the form of a Venn diagram (). These pathways are hierarchically set up, in the following order: the mating pathway at the top, then the bud scar pathway, followed by the reaction−diffusion pathway and finally the actin pathway. In short, their function boils down to the act of condensing the GTPase Cdc42 bound to GTP molecules (i.e., active Cdc42) to one point on the plasma membrane, which can signal downstream effectors to proceed the cell cycle [
29]. To prevent premature or overdue localization of active Cdc42 and allow some influence on the hierarchy of pathways, a fifth pathway exists to control the previous four, namely the timing pathway. The next section summarizes the most important interactions in and across all pathways, starting with the timing cue. As a site note, we have done our best to include all relevant papers, but apologize for important papers we have missed.
2.1. Timing: The Control Knob
During isotropic growth in G1, active Cdc42 localization is suppressed by overactivity of its associated GTPase activating proteins (GAPs) and sequestration of its guanine nucleotide exchange factor (GEF). The consequence of both circumstances is the vast abundance of inactive Cdc42, which is bound to GDP instead of GTP [
30], rendering it impossible to signal the polarity cue. The purpose of this pathway (see top dark purple region in ) is hence to timely reduce GAP activity and release the GEF, which must be in response to important physiological parameters that indicate the readiness of the cell: sufficient protein production, a sufficient size, and sufficient nutrition.
The physiological state of the cell enters the equation through nuclear levels of cyclin Cln3. Upon sufficient nutrition and size, Cln3 levels rise either more directly through higher Cln3 mRNA abundance [
31], or more indirectly through Ydj1 disturbing Cln3 localization by Whi3 [
32], the latter also being an inhibitor of Cln3 mRNA translation [
33]. The arrival of nuclear Cln3 allows binding partner and cyclin-dependent kinase Cdc28 [
34] to phosphorylate Whi5, which had inhibited expression of Cln2, another cyclin [
35,
36]. Cln2 can then reinforce its own expression, consolidating the original Cln3 signal [
37].
Now, the Cdc28-Cln2 complex can distribute the physiological signal to the aforementioned targets, the GAPs and the GEF. The kinase Cdc28 phosphorylates all four GAPs Bem2, Bem3, Rga1 and Rga2 [
38,
39,
40,
41] and Far1, which was keeping the GEF Cdc24 in the nucleus [
42]. Now cytoplasmic levels of active Cdc42 can rise, leading to polarity establishment through subsequent pathways.
Importantly, the completion of the timing pathway causes the hierarchy of the subsequent pathways to change. While the mating pathway is otherwise dominant, the kinase Cdc28 phosphorylates Ste5, a crucial hub in the mating pathway, to stop the mating in its tracks [
43,
44,
45]. In the following discussion of the mating pathway, the situation is considered where the timing pathway did not overwrite its behavior.
2.2. Mating: Heavily Cross-Linked
The mating pathway is the dominant force across the four symmetry-breaking pathways. While polarization in a random orientation is possible after the timing cue (see the section on reaction−diffusion further on), the presence of pheromones of the opposite mating type (a or α) should redirect the Cdc42 localization to the side of the pheromone signal. This process revolves around Ste5, as also depicted in the left, blue-grey circle of .
Briefly put, once pheromones bind membrane proteins Ste2 and Ste3 [
46,
47], Ste4 is released from the membrane [
48,
49] and binds Ste20 and scaffold Ste5. This scaffold binds Ste7, Ste11 and Fus3, which are activated by sequential phosphorylation [
50,
51,
52]. Fus3 may inhibit the GAPs Bem2 and Bem3 [
39], while Ste5 binds the GEF Cdc24 [
53], replacing the absence of the timing pathway result to stimulate activity of Cdc42.
While this simplified view would suffice to redirect the Cdc42 localization, the mating pathway is much more intertwined with the other pathways than seemingly necessary, particularly with the actin pathway. The abundant mechanistic redundancies result in a more complex picture, obfuscating the role of the proteins involved. For example, active Cdc42 stimulates Ste11 phosphorylation/activation [
54]. Another form of positive feedback, as well as a bridge to the actin pathway, is the Cdc42 recruitment of formin Bni1 [
55]. The resulting nucleation of actin cables may transport Ste5-GEF Cdc24 complexes [
56], possibly also through Bem1. This scaffold co-immunoprecipitates with Act1 [
57] and Far1 [
58], which is bound to the GEF Cdc24 [
59], but is itself in turn also bound to Bem1 [
60]. Another actin cross-link is the phosphorylation and localization of Bni1 through Fus3 [
61]. Clearly, care must be taken in assigning roles to different proteins, as many are overloaded.
2.3. Bud Scar: Mostly Modular and Ordered
In the absence of a mating cue, the timing pathway reduces GAP activity and releases the GEF, while the mating pathway is repressed. Under the new hierarchy, the bud scar pathway is normally dominant. The scar refers to leftover proteins from the previous division, named septins [
62,
63]. This spatial cue can be exploited for polarity establishment; a new bud forms adjacent to the scar (axial budding, haploids) or also at the opposing side (bipolar budding, diploids) [
64,
65]. The bottom, dark blue circle of represents this path from septins to Cdc42 recruitment graphically.
An important bud scar localization target is Bud5, which activates and recruits Bud1 [
66,
67], a protein also known as Rsr1 [
65,
68]. In haploids, where Axl1 is specifically expressed [
69], the Bud5 localization occurs by relay of the septin signal to a protein complex of Bud3, Bud4, Axl1, Axl2 and Bud5 [
70,
71,
72,
73]. In diploids, functionality of Rax1 and Rax2 is not impaired, presumably by blocked expression of Axl1 [
74], so these can localize Bud9 and Bud8 to the bud scar or the opposite end of the scar respectively [
75], which in turn recruit Bud5 [
76].
After Bud5, localization follows of Bud2 [
77], the GAP for Bud1 [
78], to complete the control of the GTPase cycle of Bud1. Finally, Bud1 binds GEF Cdc24 and Bem1 [
79] (although Cdc24 has the strongest affinity with Bem1 [
80]), to redirect the pattern formation made possible after the timing cue. As linkage of Cdc42 GAP Rga1 to septins prevents reuse of the previous location [
81], the new bud forms adjacent to the bud scar.
As a whole, the bud scar pathway is not completely modular either. Aside from nudging the reaction−diffusion pathway (see next section), an example of a cross-link is that Bud8 and Bud9 are delivered by actin transport [
82]. The highest position in the hierarchy in the absence of the mating cue is also not absolute; multiple ways to promote the subsequent reaction−diffusion pathway exist, such as deletion of Bud1 and Bud8 [
83], Axl2 and Rax1 [
84], or Bem1 [
22].
2.4. Reaction−Diffusion: Ample Redundancy
Even in the absence of chemical or spatial cues, the shift in balance towards activation of Cdc42 induced by the timing pathway still provides the conditions for swift symmetry breaking. Theoretical models concerning this pathway have been subject to updates as more molecular details have been revealed. Central is the strong positive feedback generated by the Bem1-GEF Cdc24 complex, as modelled in, e.g., [
85,
86] with further refinement in [
87]. This feedback is sufficient for polarity success, which becomes rather insensitive to GAP abundance. More details on the GAPs were uncovered in [
23].
What makes this pathway special is the limited number of proteins that are unique to this pathway, as seen from the central, emerald circle in , namely only Cla4 and Rdi1. The latter is the least cross-linked of the two, providing a possible justification for referring to the WT mechanism as the Rdi1 polarity mechanism, as in [
24]. Cla4 is more context-dependent, possibly having two opposing roles, promoting and inhibiting polarity [
23,
88,
89]. Yet both Rdi1 and Cla4 are dispensable for polarity [
88,
90].
An even stronger addition to the redundancy within this pathway is on the positive feedback side. Without Bem1, generic rescuing feedbacks suffice [
23], among which Cla4 could account for 20% of their function [
91]. More feedbacks may be found in the GAPs through actin transport as described in, e.g., [
24]. This brings us to the actin pathway as the final layer to discuss.
2.5. Actin: The Mysterious Auxiliary Layer
The actin pathway (rightmost green circle in ) has featured several times already in the previously discussed pathways, but its individual role is still quite uncertain. Yeast formin Bni1 which nucleates actin cables, binds active Cdc42 [
55], and is known to be involved in exo- and endocytosis [
92,
93]. This suggests transport of polarity proteins from and to the presumptive bud site. The resulting actin pathway has been confusingly implicated in two opposing roles; promoting Cdc42 polarization, see, e.g., [
24,
94,
95], as well as negatively impacting Cdc42 polarization [
96,
97].
A way to reconcile these findings is that actin transport contributes to a process promoting Cdc42 polarization but without relying on significant transport of Cdc42 itself. As mentioned in the mating pathway, Bem1 and Act1 co-immunoprecipitate [
57], suggesting that Bem1, and concordantly its multiple binding partners, might get transported through the actin pathway. However, in the absence of Bem1, 80% of the positive feedback is still unidentified [
91], which may very well be actin-related. Instead, a prime candidate is the GAP group, which is known to bind the epsin-coating of actin cables involved in endocytosis [
98]. In any case, it is quite difficult to decipher the actin pathway, in large part due to its low positioning in the yeast polarity hierarchy.