The use of deep eutectic solvents (DES) is on the rise worldwide because of the astounding properties they offer, such as simplicity of synthesis and utilization, low-cost, and environmental friendliness, which can, without a doubt, replace conventional solvents used in heaps.
- deep eutectic solvents
- extraction
- organic compounds
- green sample preparation
- analytical chemistry
1. Introduction
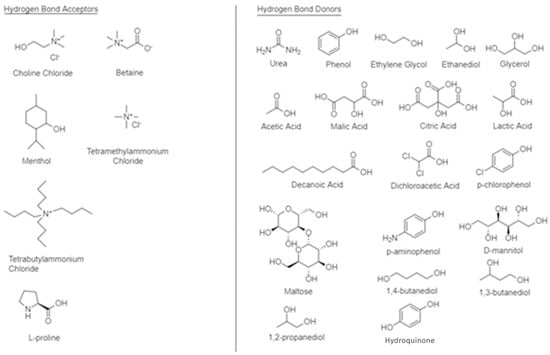
2. Synthesis of Deep Eutectic Solvents
|
Type |
Formula |
Terms |
|---|---|---|
|
I |
Cat+X− + zMClx |
M = Zn, Sn, Ga, In, Al, Fe |
|
II |
Cat+X− + zMClx · yH2O |
M = Cr, Cu, Fe, Ni, Co |
|
III |
Cat+X− + zRZ |
Z = COOH, CONH2, OH |
|
IV |
MClx + RZ |
M = Al, Zn and Z = CONH2, OH |
3. Properties of Deep Eutectic Solvents
4. Extraction of Organic Compounds
In Table 2, the methods developed for the extraction of the organic compounds from pesticides, fungicides, herbicides, flavonoids, phenolic and other bioactive compounds are summarized and presented.
Table 2. Summary and analytical performance of the methods discussed.
|
Group of Compounds |
DES |
Molar Ratio |
Extraction Technique |
Recovery (%) |
LOD 1 |
Reference |
|---|---|---|---|---|---|---|
|
Triazole fungicides |
ChCl: 4-chlorophenol |
1:2 |
HS-SDME |
94–97 |
0.82–1.0 mg/L |
[41] |
|
Pesticides |
Menthol: dichloroacetic acid |
1:2 |
DLLME |
56–86 |
0.32–1.2 ng/g |
[42] |
|
Pesticides |
ChCl: p-chlorophenol |
1:2 |
LPME |
56–93 |
0.13–0.31 ng/mL |
[43] |
|
Neonicotinoid pesticides |
Menthol: dodecanoic acid |
2:1 |
LLE |
80 |
N/R 2 |
[44] |
|
Pesticides |
ChCl: decanoic acid |
1:2 |
DLLME |
64–89 |
0.9–3.9 ng/mL |
[45] |
|
Acidic pesticides |
ChCl: ethylene glycol |
1:2 |
DLLME-SBSE |
76–90 |
7–14 ng/L |
[47] |
|
Pesticides |
TBAC: dichloroacetic acid |
1:1 |
DLLME |
81–94 |
0.09–0.27 ng/mL |
[48] |
|
Fungicides |
Menthol: decanoic acid |
1:1 |
UA-DLLME |
72–109 |
0.75–8.45 μg/mL |
[49] |
|
Herbicides |
ChCl: butyric acid |
1:2 |
LLE-DLLME |
70–89 |
2.6–8.4 ng/kg |
[50] |
|
Flavonoids |
ChCl: 1,4-butanediol |
1:5 |
UAE |
N/R |
0.07, 0.09 μg/mL |
[56] |
|
Flavonoids |
Glycerol: L-proline |
2:5 |
UAE, SPE |
81–87 |
N/R |
[57] |
|
Isoflavones |
ChCl: citric acid |
1:1 |
UAE |
64.7–99.2 |
0.06–0.14 μg/mL |
[58] |
|
Flavonoids |
ChCl: 1,2-propanediol |
1:4 |
UAE |
86.7–98.9 |
0.05–0.14 μg/mL |
[59] |
|
Flavonoids |
ChCl: oxalic acid: ethylene glycol |
1:1:3 |
LLE |
N/R |
1.11–1.40 mg/g |
[60] |
|
Flavonoids |
ChCl: acetic acid |
1:2 |
UAE |
N/R |
1.11–11.57 mg/g |
[61] |
|
Flavonoids |
Betaine: D-mannitol |
N/R |
UAE |
91.7–95.8 |
0.14–0.17 μg/mL |
[62] |
|
Flavonoids |
Different types |
Diff. 3 |
UAE |
93.8–107.7 |
0.14–0.22 μg/mL |
[63] |
|
Bioactive compounds |
ChCl: malic acid |
1:1 |
UAE |
N/R |
N/R |
[64] |
|
Bioactive compounds |
ILs, ChCl based DES |
Diff. |
Reflux |
N/R |
N/R |
[65] |
|
Anthocyanins |
ChCl: glycerol:citric acid |
0.5:2:0.5 |
UAE |
N/R |
0.02 mg/L |
[66] |
|
Anthocyanins |
ChCl: malic acid |
Diff. |
UAE |
N/R |
0.15–0.28 mg/L |
[67] |
|
Anthocyanins |
Lactic acid: glucose |
1:2 |
Stirring |
N/R |
N/R |
[68] |
|
Phenolic metabolites |
Different types |
Diff. |
N/R |
75–97 |
N/R |
[71] |
|
Phenolics |
ChCl: oxalic acid |
1:1 |
MAE, UAE |
N/R |
0.05–0.37 mg/L |
[72] |
|
Phenolics |
ChCl: 1,2-propanediol |
1:1 |
Vortex |
N/R |
N/R |
[73] |
|
Phenolics |
ChCl: lactic acid |
1:2 |
MAE, HUE, UAE |
N/R |
N/R |
[74] |
|
Phenolics |
ChCl + phenolics |
N/R |
RDSE |
66–87 |
10–60 μg/L |
[75] |
|
Polyphenols |
ChCl: ethylene glycol |
1:4 |
SLE |
N/R |
N/R |
[77] |
|
Phenolics |
Lactic acid: glucose |
5:1 |
UAE |
86.0–109.6 |
0.6–89.1 ng/g |
[78] |
|
Polyphenols |
Lactic acid: glucose |
5:1 |
SLE |
N/R |
N/R |
[79] |
|
Phenolics |
ChCl: malic acid |
1.5:1 |
P-UAE |
N/R |
N/R |
[80] |
|
Phenolics |
Glycerol: citric acid: glycine |
Diff. |
UAE |
N/R |
N/R |
[81] |
|
Flavonoids |
ChCl: glycerol |
1:1 |
UAE |
95 |
N/R |
[82] |
|
Polyphenols |
Lactic acid: glycine:water |
3:1:3 |
UAE |
N/R |
N/R |
[84] |
|
Phenolic acids |
ChCl: 1,3-butanediol |
1:6 |
MAE |
79.2–86.0 |
N/R |
[85] |
|
Phenolics |
ChCl: glycerol |
1:2 |
MAE |
77.8–83.8 |
0.15–0.78 μg/mL |
[86] |
|
Phenolics |
Tetramethyl ammonuium chloride:urea |
1:4 |
UAE |
97.3–100.4 |
N/R |
[87] |
|
Flavanones |
Betaine: ethanediol |
1:4 |
Heated LLE |
97.0–101.6 |
N/R |
[88] |
|
Bioactive compounds |
ChCl: propylene glycol |
1:1 |
UAE |
N/R |
N/R |
[89] |
|
Phenolics |
ChCl: acetic acid |
1:2 |
Thermo-shaking |
N/R |
N/R |
[90] |
|
Phenolics |
TBAC: hydroquinone |
1:2 |
LLE/DLLME |
74–89 |
0.13–0.42 ng/mL |
[91] |
|
Phenols |
L-proline: decanoic acid |
1:4.2 |
Stirring |
57–62 |
N/R |
[92] |
1 Limit of Detection, 2 Not reported, 3 Different molar ratios tested.
|
Group of Compounds |
DES |
Molar Ratio |
Extraction Technique |
Recovery (%) |
LOD 1 |
Reference |
|---|---|---|---|---|---|---|
|
Sulfonamide |
ChCl: ethylene glycol |
1:2 |
PT-SPE |
91.0–96.8 |
0.01 μg/mL |
[93] |
|
Analgetic |
Betaine: oxalic acid |
1:2 |
SA-DES-ME |
94.2–107.1 |
14.9 μg/L |
[94] |
|
Antibiotics |
ChCl: glycerol |
1:2 |
MIPs-SPE |
87.0–91.2 |
N/R 2 |
[95] |
|
Antivirals |
TBAC: p-aminophenol |
1:2 |
LLE |
90–96 |
1.0–1.3 μg/L |
[96] |
|
Antimalarial |
Menthol: fenchyl alcohol |
1:1 |
Stirring |
101 |
N/R |
[97] |
|
NSAID 3 |
Menthol: acetic acid |
Diff. 4 |
LLE |
80 |
N/R |
[98] |
|
Antibiotics |
TBAC: butanol |
1:1 |
SPE, DLLME |
84–99 |
0.32–0.86 ng/g |
[99] |
|
Tocotrienols, tocopherols |
ChCl: malonic acid |
1:1 |
LLE |
93.0–99.8 |
N/R |
[100] |
|
α-tocopherols |
ChCl: sucrose |
1:2 |
LLE |
N/R |
N/R |
[101] |
|
α-, γ-, δ-tocopherols |
ChCl: p-cresol |
1:2 |
Vortex |
77.6 |
N/R |
[102] |
|
Parabens |
Menthol: decanoic acid |
2:1 |
LLME |
69.1–78.5 |
0.6–0.8 mg/mL |
[103] |
|
PAHs |
Menthol or thymol with fatty acids |
Diff. |
Stirring |
70–91 |
2–90 ng/kg |
[105] |
|
VOCs |
ChCl: urea |
1:3 |
MAE, HS-SPME |
N/R |
N/R |
[106] |
|
Polysaccharides |
ChCl: 1,4-butanediol |
N/R |
UAE |
N/R |
N/R |
[107] |
|
Polysaccharides |
ChCl: 1,4-butanediol |
1:5 |
MAE |
91.2 |
N/R |
[108] |
|
Polysaccharides |
ChCl: 1,2-propanediol |
1:2 |
UAE |
N/R |
N/R |
[109] |
|
Polysaccharides |
ChCl: glycerol |
1:2 |
Thermal treatment |
60.3 |
N/R |
[110] |
|
Natural pigments |
Citric acid: glucose |
1:1 |
SPE |
88.5–94.4 |
0.25–0.37 mg/L |
[111] |
|
Curcuminoids |
ChCl: lactic acid |
1:1 |
UAE |
N/R |
N/R |
[112] |
|
Curcuminoids |
ChCl: phenol |
1:4 |
VA-LLME |
96–102 |
2.86 μg/L |
[113] |
|
Pigment |
Different types |
Diff. |
UAE |
N/R |
N/R |
[114] |
|
Pigment |
ChCl: ethylene glycol |
Diff. |
N/R |
N/R |
N/R |
[115] |
|
Terpene trilactones |
Betaine: ethylene glycol |
Diff. |
UAE |
99.4 |
N/R |
[116] |
|
Benzophenone-type UV filters |
Menthol: decanoic acid |
1:1 |
AA-DLLME |
88.8–105.9 |
0.05–0.2 ng/mL |
[117] |
|
Organic solvents |
Menthol: capric acid |
2:1 |
LLE |
N/R |
N/R |
[118] |
|
Tanshinones |
ChCl: -1,3-butanediol |
N/R |
BMAE |
96.1–103.9 |
5–8 ng/mL |
[119] |
|
Catechins |
Different types |
Diff. |
LLE |
82.7–97.0 |
N/R |
[120] |
|
Essential oils |
ChCl: L-lactic acid |
1:3 |
MAE |
N/R |
N/R |
[121] |
|
Essential oils |
ChCl: oxalic acid |
1:1 |
MAE |
N/R |
N/R |
[122] |
|
Steviol glycosides |
Tetraethylammonium chloride: ethylene glycol |
1:2 |
UAE |
N/R |
N/R |
[123] |
1 Limit of Detection, 2 Not reported, 3 Non-steroidal anti-inflammatory drugs, 4 Different molar ratios tested.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27227699
