Fish die-offs are important indicators of aquatic environmental problems, and although some fish species are very sensitive to adverse changes in environmental conditions (there are many fish species that have a relatively low tolerance to changes in the environment), it is important to remember that such changes usually affect entire aquatic ecosystems, and thus other animals and plants, as well as everything related to the bottom life of the aquatic environment. Localized sudden and mass fish kills or even whole fish populations and deterioration (mortality) in aquatic life in different types of water bodies, namely freshwater, marine, and estuarine, have been observed quite frequently and excessively. Although the causes of their occurrence may be natural, anthropogenic changes and pollution (including toxins) in aquatic and terrestrial systems are major contributors to the increasing frequency and magnitude of fish kills worldwide.
- harmful algal blooms
- ecological catastrophe
- prymnesins
- river ecosystem
- aquatic toxicity
- water pollution
- environmental monitoring
- eutrophication
- microalgal ecology
- fish kill
1. Toxins
2. Algae Blooms and Harmful Toxins Secreted by Habitats of Algal Blooms
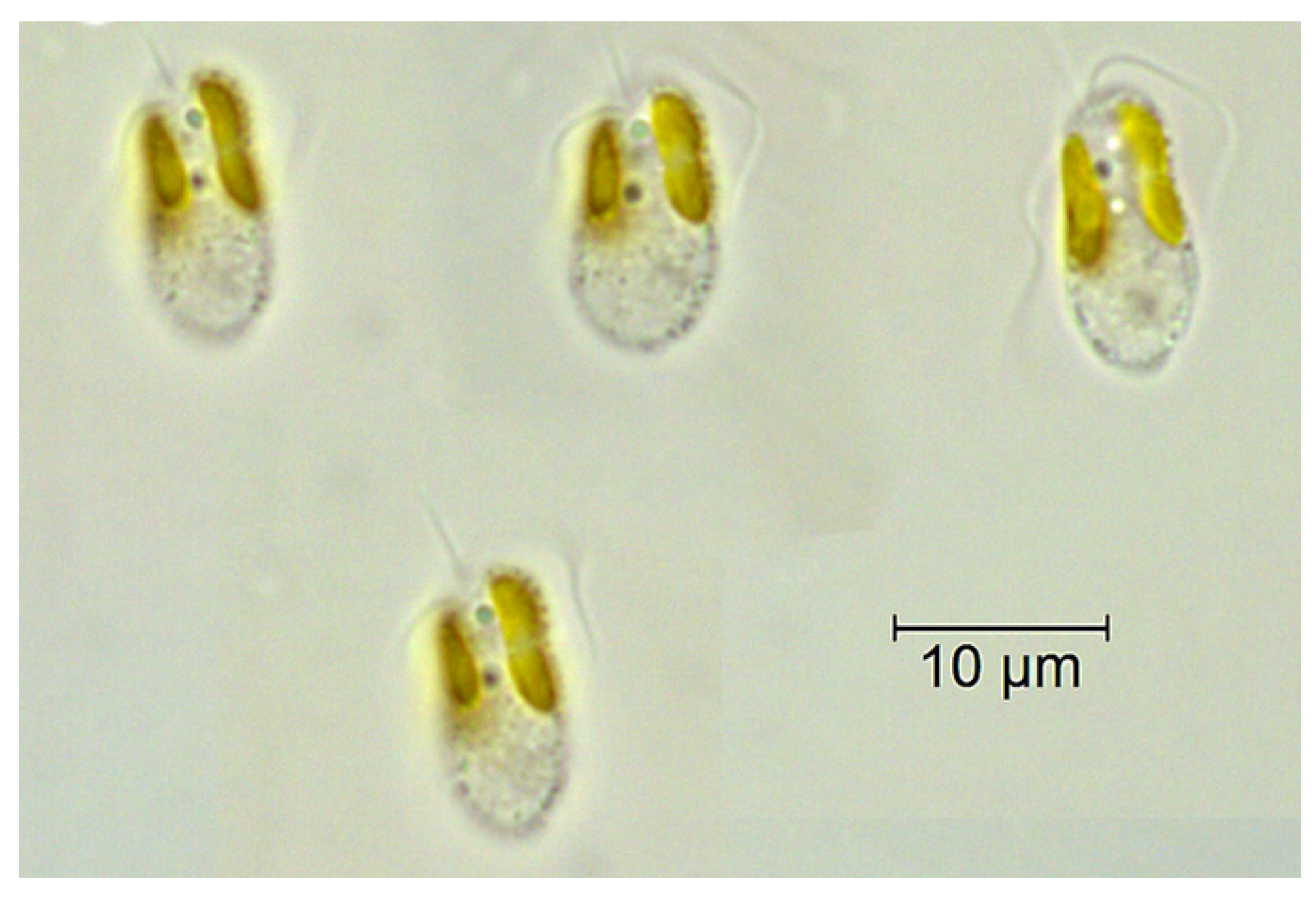
| Case Study Description | Country/Authors |
|---|---|
| In 2009, a devastating P. parvum bloom occurred at Barramundi Farm in Australia, resulting in the loss of all fish in the ponds. In response, a number of measures were taken to prevent similar incidents in the future [73]. To this end, an experimental manipulation of nutrients and pH was conducted in one of the ponds. The experimental pond was treated with Phoslock™ clay modified with lanthanum cations that irreversibly bind dissolved phosphorus in the water, and the pH was lowered to below 7.7 by adding molasses, which stimulates microbial growth. Despite these preventive measures, a bloom of P. parvum occurred in the culture ponds at water temperatures of 24 to 32 °C and salinities of 10 to 36 ppt, resulting in the death of all fish. | Australia: Seger et al. [73] |
| According to Guo et al. [74], citing numerous Chinese authors, there are three prymnesium species in China: P. parvum, P. saltans, and P. papillarum. In 1963, a phytoplankton bloom caused by P. parvum resulted in the loss of 100,000 carp fry in a fish farm at the Liaoning Province Fishery College in Dalian. Since then, this alga has been found every year in different parts of China, such as Tianjin, the Ningxia Autonomous Region, Inner Mongolia, Shanxi and Zhejiang provinces, and the Tibet Autonomous Region. P. parvum thrives in coastal brackish water in Dalian, Tianjin, and Zhejiang and in saline, sulfate-bearing inland water in Ningxia, Inner Mongolia, and Shanxi. P. saltans, on the other hand, has been isolated from the coasts of Guangdong, Tianjin, and Ningxia and occurs in similar habitats and locations as Ningxia. P. papillarum has been isolated from the coast of Shandong Province. Chen and Zeng [75] described a new species in China that caused rotifers and copepods that fed on it to die within 2 to 4 days [74]. | China: Guo et al. [74]; Chen and Zeng [75] |
| In 2007, an increase in the occurrence of the alternative algal species Prymnesium polylepis was detected during a marine monitoring program [76]. This species is considered the second most important ichthyotoxic haptophyte after P. parvum. The peak of the extensive bloom occurred between March and May 2008 and was widespread in the southern, central, and northwestern Baltic Sea, with cell concentrations reaching up to 5 million cells per liter. At some sites, P. polylepis accounted for 30 to 90% of the total phytoplankton volume. However, in the northeastern Baltic Sea and the Gulf of Finland, P. polylepis was detected in low numbers. Larsson et al. [76] studied the effects of this extensive bloom on duck-billed birds, i.e., eiders (Somateria mollissima), in the Baltic Sea. They observed a sharp decline in breeding eiders at 28 colonies in the southern and central Baltic Sea between 2007 and 2008. The authors argue that the intense spring bloom of P. polylepis affected the eiders’ main food source, i.e., mussels, at feeding sites in both toxic and non-toxic ways, which affected the body condition of adult female eiders and their breeding readiness. | Denmark: Larsson et al. [76] |
| In 1990, Lindholm and Virtanen reported a bloom of P. parvum in the Strait of Dragsfjaerd in Finland that resulted in a fish kill. The concentration of P. parvum reached a peak of 50,000 cells/mL. Chemical analyses conducted during the disappearance of the bloom showed a decrease in total phosphorus and total nitrogen levels and a decrease in chlorophyll a levels. Levels were about 50% lower in areas outside the strait where P. parvum was present in lower numbers. Live cells of P. parvum showed autofluorescence that could be of diagnostic value. Seven years later, in 1997, a brackish water lake in SW Finland, Vargsundet, was contaminated with algal toxins, resulting in high fish mortality. During this event, dense populations of Prymnesium sp. and the toxin-producing cyanobacterium Planktothrix agardhii were observed, mainly in separate layers [77]. | Finland: Lindholm and Virtanen [77] |
| Great Britain is historically significant because it is the country where a case of P. parvum was first documented (according to Carter’s 1937 publication). More specifically, P. parvum habitats were found in a brackish pond near Bembridge on the Isle of Wight. In the 1960s, reports of the species’ occurrence surfaced in Hickling Broad, part of the Norfolk Broads. The Norfolk Broads, which consist of shallow brackish water created by centuries of peat extraction, are now used for recreational purposes and generate an estimated GBP 550 million in annual revenue for the local economy. According to Wagstaff et al. [78], P. parvum blooms have likely been present in the Norfolk Broads since the early 20th century. The frequency of blooms in the region is high, as evidenced by the toxic P. parvum bloom in Hickling Broad in 2015 that resulted in the death of thousands of fish. To contain the damage, 600,000 fish were manually relocated and rescued [79]. | Great Britain: Wagstaff et al. [78]; Wagstaff et al. [79]; |
| According to Shilo and Shilo [80], P. parvum first appeared in Israel in 1947 and then spread rapidly in brackish water areas, causing major problems for fish farming. The authors argue that the control of P. parvum can be achieved either by destroying the organism or its toxin. In 1947, Reich and Aschner discovered that ammonium sulfate had a destructive effect on P. parvum even at low concentrations and was not harmful to other life forms. Gordon and Colorni [81] reported a bloom of P. parvum in the Arava Valley in southern Israel that resulted in gradual death of ornamental fish, Poecilia sp. and koi. The toxic effect was due to changes in the water system, including higher temperatures, a tripling in salinity, and eutrophication, which favored the growth of P. parvum. Treatment with 10 ppm ammonium sulfate stopped the fish kill. | Israel: Shilo and Shilo [80]; Gordon and Colorni [81] |
| In 1990, a significant algal bloom and fish kill were observed in the Botshol Reserve in Utrecht, the Netherlands, which consists of two shallow lakes, ditches, and reed beds. The reserve was originally a system of clear lakes, but due to eutrophication, the water quality has deteriorated since the 1960s. Efforts were made to restore the reserve by reducing external phosphorus inputs. This resulted in a significant reduction in phosphorus levels in the lake water, an improvement in the light climate, and a change in the composition of phyto- and zooplankton. However, these measures also led to the appearance of P. parvum blooms [82]. | Netherlands: Rip et al. [82] |
| In Norway, the occurrence of P. parvum has been documented along the entire west coast, from Oslofjord in the south to Spitsbergen in the north. However, blooms of the species have only been observed in the Sandsfjord fjord system, which is characterized by a permanent brackish water layer at a depth of 2–5 m and a summer salinity of 4–7 psu. The first recorded bloom of P. parvum in Sandsfjord occurred in late July 1989 and had severe consequences for local fish farms. According to Johnsen et al. [83], the bloom resulted in the death of 750 tons of Atlantic salmon and rainbow trout. In subsequent years, blooms of P. parvum occurred repeatedly in July and August, resulting in losses to salmon farms. Due to the continued bloom, fish farmers finally decided to leave the area in 1995, which marked the end of the bloom. However, in 2005, an attempt to return to the region to farm fish resulted in another P. parvum bloom in 2007 and the loss of 135 tons of salmon. | Norway: Johnsen et al. [83] |
| The spread of the harmful P. parvum algae has caused major fish kills and financial losses in Texas and throughout the United States. According to Texas Parks and Wildlife agencies, fish kills in the upper Brazos River in 1981–1982 and in Red Bluff Reservoir in 1985 were likely caused by P. parvum, although this has not been confirmed. In 1985, P. parvum was confirmed as the cause of a 660 km fish kill in the Pecos River that resulted in the death of an estimated 110,000 fish. Between 1985 and 2000, P. parvum blooms caused fish kills in the Brazos, Colorado, and Rio Grande watersheds, killing an estimated 2.6 million fish. In the early 2000s, the rapid spread of P. parvum led to blooms in 15 other U.S. states, including Alabama, Arizona, and California. Now, the alga is present in all southern regions of the country and in some northern regions [56]. In 2001, a bloom of P. parvum caused significant damage to Texas fisheries. A winter bloom caused a massive fish kill in Lake Possum Kingdom and other reservoirs, as well as the death of over 5 million striped and hybrid perch fry in the Wichita River. In 2003, P. parvum invaded the Canadian River watershed and caused a minor fish kill. In subsequent years, more than 30 watersheds were affected by the alga. It is estimated that the P. parvum bloom caused the mortality of more than thirty-four million fish and caused tens of millions of dollars in financial losses [56]. Cases of P. parvum have been reported throughout the decade 2011–2020 in several regions of the United States [84][85], including Brady Creek Reservoir (2012), Colorado City Reservoir (2016), Concho River (2019), Baylor Creek Reservoir (2009, 2012), Buffalo Springs Reservoir (2011–2012, 2014–2016), Balmorhea Revervoir (2010), Diversion Reservoir—Lake Diversion (2010–2016), and in Southern California, in Lake Forest and Lake Elsinore (2014). Detailed information can be found in the Nonindigenous Aquatic Species Database [84]. | United States: Roelke et al. [56]; Nonindigenous Aquatic Species Database [84]; Caron [85] |
This entry is adapted from the peer-reviewed paper 10.3390/toxins15060403
References
- Baatrup, E. Structural and functional effects of heavy metals on the nervous system, including sense organs, of fish. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1991, 100, 253–257.
- Vilariño, N.; Louzao, M.C.; Abal, P.; Cagide, E.; Carrera, C.; Vieytes, M.R.; Botana, L.M. Human poisoning from marine toxins: Unknowns for optimal consumer protection. Toxins 2018, 10, 324.
- Arcand-Hoy, L.D.; Benson, W.H. Fish reproduction: An ecologically relevant indicator of endocrine disruption. Environ. Toxicol. Chem. Int. J. 1998, 17, 49–57.
- Liu, G.; Ke, M.; Fan, X.; Zhang, M.; Zhu, Y.; Lu, T.; Qian, H. Reproductive and endocrine-disrupting toxicity of Microcystis aeruginosa in female zebrafish. Chemosphere 2018, 192, 289–296.
- Rojas-Hucks, S.; Rodriguez-Jorquera, I.A.; Nimpstch, J.; Bahamonde, P.; Benavides, J.A.; Chiang, G.; Galbán-Malagón, C.J. South American National Contributions to Knowledge of the Effects of Endocrine Disrupting Chemicals in Wild Animals: Current and Future Directions. Toxics 2022, 10, 735.
- Segner, H.; Bailey, C.; Tafalla, C.; Bo, J. Immunotoxicity of xenobiotics in fish: A role for the aryl hydrocarbon receptor (AhR)? Int. J. Mol. Sci. 2021, 22, 9460.
- Segner, H.; Rehberger, K.; Bailey, C.; Bo, J. Assessing fish immunotoxicity by means of in vitro assays: Are we there yet? Front. Immunol. 2022, 13, 835767.
- Taslima, K.; Al-Emran, M.; Rahman, M.S.; Hasan, J.; Ferdous, Z.; Rohani, M.F.; Shahjahan, M. Impacts of heavy metals on early development, growth and reproduction of fish—A review. Toxicol. Rep. 2022, 9, 858–868.
- Dao, H.V.; Uesugi, A.; Uchida, H.; Watanabe, R.; Matsushima, R.; Lim, Z.F.; Suzuki, T. Identification of fish species and toxins implicated in a snapper food poisoning event in Sabah, Malaysia, 2017. Toxins 2021, 13, 657.
- EPA. Toxics in the Food Web; Environmental Protection Agency: Washington, DC, USA, 2021.
- Bhuyan, M.S. Effects of microplastics on fish and in human health. Front. Environ. Sci. 2022, 10, 250.
- Allaf, M.M.; Trick, C.G. Yeast Cell as a Bio-Model for Measuring the Toxicity of Fish-Killing Flagellates. Toxins 2021, 13, 821.
- Sharma, L.; Siedlewicz, G.; Pazdro, K. The toxic effects of antibiotics on freshwater and marine photosynthetic microorganisms: State of the art. Plants 2021, 10, 591.
- Keena, M.; Meehan, M.; Scherer, T. Environmental Implications of Excess Fertilizer and Manure on Water Quality; North Dakota State University: Fargo, ND, USA, 2022.
- Hargreaves, J.A.; Tucker, C.S. Managing Ammonia in Fish Ponds; Southern Regional Aquaculture Center: Stoneville, NC, USA, 2004; Volume 4603.
- Camargo, J.A.; Alonso, Á. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849.
- Nicholas, W.; Brye, B.A. Experience with Heavy Metals in the Tennessee Valley Authority System: Proceedings of the International Conference Held in Nashville, Tennessee, USA, December 1973; Pergamon Press: London, UK, 1973.
- Bu-Olayan, A.H.; Thomas, B.V. Validating mercury levels in catfish Netuma thalassina (Rüppell, 1837) during and aftermath ‘fish kill’in Kuwait Bay. Iran. J. Fish. Sci. 2020, 19, 3151–3159.
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Li, S. Health and environmental effects of heavy metals. J. King Saud Univ. Sci. 2022, 34, 101653.
- EPA. Polychlorinated Biphenyls (PCBs) Update: Impact on Fish Advisories; Environmental Protection Agency: Washington, DC, USA, 1999.
- Duke, T.W.; Lowe, J.I.; Wilson, A.J. A polychlorinated biphenyl (Aroclor 1254) in the water, sediment, and biota of escambia bay, Florida. Bull. Environ. Contam. Toxicol. 1970, 5, 171–180.
- Nestel, H.; Budd, J. Chronic oral exposure of rainbow trout (Salmo gairdneri) to a polychlorinated biphenyl (Aroclor 1254): Pathological effects. Can. J. Comp. Med. 1975, 39, 208.
- Henry, T.B. Ecotoxicology of polychlorinated biphenyls in fish—A critical review. Crit. Rev. Toxicol. 2015, 45, 643–661.
- Eisler, R.; Wiemeyer, S.N. Cyanide hazards to plants and animals from gold mining and related water issues. Rev. Environ. Contam. Toxicol. 2004, 183, 21–54.
- National Research Council Committee. Oil in the Sea: Inputs, Fates, and Effects; National Academies Press: Washington, DC, USA, 2003.
- Barron, M.G.; Vivian, D.N.; Heintz, R.A.; Yim, U.H. Long-term ecological impacts from oil spills: Comparison of Exxon Valdez, Hebei Spirit, and Deepwater Horizon. Environ. Sci. Technol. 2020, 54, 6456–6467.
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1.
- EPA. Protecting Water Quality from Agricultural Runoff; Environmental Protection Agency: Washington, DC, USA, 2005.
- U.S. National Office for Harmful Algal Blooms. Examples of Socioeconomic Impacts. Harmful Algae. Available online: https://hab.whoi.edu/impacts/impacts-socioeconomic/ (accessed on 20 February 2023).
- Sha, J.; Xiong, H.; Li, C.; Lu, Z.; Zhang, J.; Zhong, H.; Yan, B. Harmful algal blooms and their eco-environmental indication. Chemosphere 2021, 274, 129912.
- Burkholder, J.M. Harmful algal blooms. In Encyclopedia of Inland Waters; Cary Institute of Ecosystem Studies Millbrook: New York, NY, USA, 2009; Volume 1, pp. 264–285.
- Larson, S.J.; Capel, P.D.; Majewski, M.S. Pesticides in Surface Waters: Distribution, Trends, and Governing Factors; CRC Press: Boca Raton, FL, USA, 2019; p. 278.
- Rosseland, B.O.; Blakar, I.A.; Bulger, A.; Kroglund, F.; Kvellstad, A.; Lydersen, E.; Vogt, R. The mixing zone between limed and acidic river waters: Complex aluminium chemistry and extreme toxicity for salmonids. Environ. Pollut. 1992, 78, 3–8.
- Rask, M.; Järvinen, M.; Kuoppamäki, K.; Pöysä, H. Limnological responses to the collapse of the perch population in a small lake. Ann. Zool. Fenn. 1996, 1, 517–524.
- Poléo, A.B. Aluminium polymerization—A mechanism of acute toxicity of aqueous aluminium to fish. Aquat. Toxicol. 1995, 31, 347–356.
- Francis-Floyd, R.; Riggs, A.; Philips, E. A Beginner’s Guide to Water Management—Fish Kills; University of Florida, Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2004.
- DiPinto, L.; Penn, T.; Iliff, J.; Peterson, C. Determining the Scale of Restoration for a Fish Kill in the Alafia River, FLORIDA; American Petroleum Institute: Washington, DC, USA, 2001.
- Khalidi-idrissi, A.; Madinzi, A.; Anouzla, A.; Pala, A.; Mouhir, L.; Kadmi, Y.; Souabi, S. Recent advances in the biological treatment of wastewater rich in emerging pollutants produced by pharmaceutical industrial discharges. Int. J. Environ. Sci. Technol. 2023, 20, 1–22.
- Cormier, S.M.; Suter, G.W.; Norton, S.B. Causal characteristics for ecoepidemiology. Hum. Ecol. Risk Assess. 2010, 16, 53–73.
- Fujawa, E. 100 Years of Contamination: The White River Fish Kills of 1896 and 1999. Available online: https://www.class900indy.com/post/100-years-of-contamination-the-white-river-fish-kills-of-1896-and-1999 (accessed on 17 May 2023).
- Bowman, S. 113 Tons of Dead Fish: Indiana’s Worst Environmental Disaster, 20 Years Later. Available online: https://www.indystar.com/in-depth/news/environment/2019/12/19/guide-corp-s-toxic-discharge-killed-millions-fish-white-river/4385458002/ (accessed on 17 May 2023).
- Thornhill Law Firm. Temple-Inland Pearl River Paper Mill Spill. Available online: https://www.thornhilllawfirm.com/temple-inland-pearl-river-paper-mill-spill.html (accessed on 17 May 2023).
- Mother Earth News. Manure Spills: The Environmental Damage We Don’t Hear About. Available online: https://www.motherearthnews.com/sustainable-living/nature-and-environment/environmental-damage-from-spills-ze0z1411zdeh/ (accessed on 17 May 2023).
- Kamana, L. Trapped Fish, Just One of Many Environmental Impacts Left by Flood. Available online: https://midmichigannow.com/news/local/trapped-fish-just-one-of-many-environmental-impacts-left-by-flood (accessed on 17 May 2023).
- U.S. Fish and Wildlife Service. Tittabawassee River—Saginaw River & Bay Natural Resource Trustee Councils Draft Supplemental Restoration Plan and Environmental Assessment. Available online: https://www.fws.gov/sites/default/files/documents/nrdar-draft-supplemental%20restoration%20plan-environmental%20assessment-2023-tittabawassee%20saginaw%20river.pdf (accessed on 17 May 2023).
- Wilkerson, I. Tainting of Fish Feared after Michigan Floods. Available online: https://www.nytimes.com/1986/09/24/us/tainting-of-fish-feared-after-michigan-floods.html (accessed on 17 May 2023).
- Public Sector Consultants, Inc. Measures of Success: Addressing Environmental Impairments in the Saginaw River and Saginaw Bay. Available online: https://www.baycounty-mi.gov/Docs/EACD/Measures%20of%20Success%20-%20Saginaw%20River%20and%20Bay.pdf (accessed on 17 May 2023).
- Hartig, J. Roller Coaster: Michigan’s Long History with Environmental Contamination. Available online: https://www.greatlakesnow.org/2020/06/michigan-history-environmental-contamination/ (accessed on 17 May 2023).
- Magaña, H.A.; Contreras, C.; Villareal, T.A. A historical assessment of Karenia brevis in the western Gulf of Mexico. Harmful Algae 2003, 2, 163–171.
- Castle, K.T.; Flewelling, L.J.; Bryan, J.; Kramer, A.; Lindsay, J.; Nevada, C.; Landsberg, J.H. Coyote (Canis latrans) and domestic dog (Canis familiaris) mortality and morbidity due to a Karenia brevis red tide in the Gulf of Mexico. J. Wildl. Dis. 2013, 49, 955–964.
- Butler, N.; Carlisle, J.C.; Linville, R.; Washburn, B. Microcystins. A Brief Overview of Their Toxicity and Effects, with Special Reference to Fish, Wildlife, and Livestock; Office of Environmental Health Hazard Assessment: Sacramento, CA, USA, 2009.
- Ferrão-Filho, A.D.; Kozlowsky-Suzuki, B. Cyanotoxins: Bioaccumulation and effects on aquatic animals. Mar. Drugs 2011, 9, 2729–2772.
- Kang, Y.; Gobler, C.J. The brown tide algae, Aureococcus anophagefferens and Aureoumbra lagunensis (Pelagophyceae), allelopathically inhibit the growth of competing microalgae during harmful algal blooms. Limnol. Oceanogr. 2018, 63, 985–1003.
- Watson, S.B.; Whitton, B.A.; Higgins, S.N.; Paerl, H.W.; Brooks, B.W.; Wehr, J.D. Harmful algal blooms. In Freshwater Algae of North America; Academic Press: Cambridge, MA, USA, 2015; pp. 873–920.
- Roelke, D.L.; Manning, S.R. Harmful Algal Species Fact Sheet: Prymnesium parvum (Carter) “Golden Algae”. Harmful Algal Blooms: A Compendium Desk Reference, 1st ed.; Wiley: Hoboken, NJ, USA, 2018; pp. 629–632.
- Roelke, D.L.; Barkoh, A.; Brooks, B.W.; Grover, J.P.; Hambright, K.D.; LaClaire, J.W.; Patino, R. A chronicle of a killer alga in the west: Ecology, assessment, and management of Prymnesium parvum blooms. Hydrobiologia 2016, 764, 29–50.
- Wagstaff, B.A.; Hems, E.S.; Rejzek, M.; Pratscher, J.; Brooks, E.; Kuhaudomlarp, S.; Field, R.A. Insights into toxic Prymnesium parvum blooms: The role of sugars and algal viruses. Biochem. Soc. Trans. 2018, 46, 413–421.
- Anderson, D.M.; Burkholder, J.M.; Cochlan, W.P.; Glibert, P.M.; Gobler, C.J.; Heil, C.A.; Vargo, G.A. Harmful algal blooms and eutrophication: Examining linkages from selected coastal regions of the United States. Harmful Algae 2008, 8, 39–53.
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Suddleson, M. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13.
- CDC. Harmful Algal Bloom—Associated Illnesses. Available online: https://www.cdc.gov/habs/environment.html (accessed on 26 April 2023).
- EPA. Harmful Algal Blooms. Available online: https://www.epa.gov/nutrientpollution/harmful-algal-blooms (accessed on 26 April 2023).
- NOAA. National Oceanic and Atmospheric Administration What Is Eutrophication? Available online: https://oceanservice.noaa.gov/facts/eutrophication.html (accessed on 26 April 2023).
- Kangur, K.; Kangur, A.; Kangur, P.; Laugaste, R. Fish kill in Lake Peipsi in summer 2002 as a synergistic effect of a cyanobacterial bloom, high temperature, and low water level. Proc. Estonian Acad. Sci. Biol. Ecol. 2005, 54, 67–80.
- Carter, N. New or interesting algae from brackish water. Arch. Protistenk. 1937, 90, 1–68.
- Igarashi, T.; Satake, M.; Yasumoto, T. Prymnesin-2: A potent ichthyotoxic and hemolytic glycoside isolated from the red tide alga Prymnesium parvum. J. Am. Chem. Soc. 1996, 118, 479–480.
- Larsen, A. Prymnesium parvum and P. patelliferum (Haptophyta)—One species. Phycologia 1999, 38, 541–543.
- Edvardsen, B.; Eikrem, W.; Throndsen, J.; Saez, A.G.; Probert, I.; Medlin, L.K. Ribosomal DNA phylogenies and a morphological revision provide the basis for a revised taxonomy of the Prymnesiales (Haptophyta). Eur. J. Phycol. 2011, 46, 202–228.
- Manton, I.; Leedale, G.F. Observations on the fine structure of Prymnesium parvum Carter. Archiv. Mikrobiol. 1963, 45, 285–303.
- Manton, I. Observations with the electron microscope on the division cycle in the flagellate Prymnesium parvum Carter. J. Royal Microsc. Soc. 1964, 83, 317–325.
- Moestrup, Ø.; Thomsen, H.A. Taxonomy of toxic haptophytes (prymnesiophytes). Man. Harmful Mar. Microalgae 2003, 33, 433.
- Seoane, S.; Eikrem, W.; Pienaar, R.; Edvardsen, B. Chrysochromulina palpebralis sp. nov. (Prymnesiophyceae): A haptophyte, possessing two alternative morphologies. Phycologia 2009, 48, 165–176.
- González, A.; Broce, K.; Fábrega-Duque, J.; Tejedor-Flores, N.; Young, K. Identification and monitoring of microalgal genera potentially capable of forming harmful algal blooms in Punta Galeta, Panama. Air Soil Water Res. 2019, 12, 1178622119872769.
- Seger, A.; Dorantes-Aranda, J.J.; Müller, M.N.; Body, A.; Peristyy, A.; Place, A.R.; Hallegraeff, G. Mitigating fish-killing Prymnesium parvum algal blooms in aquaculture ponds with clay: The importance of pH and clay type. J. Mar. Sci. Eng. 2015, 3, 154–174.
- Guo, M.; Harrison, P.J.; Taylor, F.J.R. Fish kills related to Prymnesium parvum N. Carter (Haptophyta) in the People’s Republic of China. J. Appl. Phycol. 1996, 8, 111–117.
- Chen, S.F.; Zeng, C.K. Two species of Prymnesium from the North China. Oceanol. Limnol. Sin. 1986, 17, 394–399.
- Larsson, K.; Hajdu, S.; Kilpi, M.; Larsson, R.; Leito, A.; Lyngs, P. Effects of an extensive Prymnesium polylepis bloom on breeding eiders in the Baltic Sea. J. Sea Res. 2014, 88, 21–28.
- Lindholm, T.; Virtanen, T. A bloom of Prymnesium parvum Carter in a small coastal inlet in Dragsfjärd, southwestern Finland. Environ. Toxicol. Water Qual. 1992, 7, 165–170.
- Wagstaff, B.A.; Pratscher, J.; Rivera, P.P.L.; Hems, E.S.; Brooks, E.; Rejzek, M.; Field, R.A. Assessing the toxicity and mitigating the impact of harmful Prymnesium blooms in eutrophic waters of the Norfolk Broads. Environ. Sci. Technol. 2021, 55, 16538–16551.
- Wagstaff, B.A.; Pratscher, J.; Rivera, P.P.L.; Hems, E.S.; Brooks, E.; Rejzek, M.; Field, R.A. Dissecting the toxicity and mitigating the impact of harmful Prymnesium blooms in the UK waters of the Norfolk Broads. bioRxiv Electron. J. 2020, 1, 2020–2023.
- Shilo, M.; Shilo, M. Conditions which determine the efficiency of ammonium sulphate in the control of Prymnesium parvum in fish breeding ponds. Appl. Microbiol. 1953, 1, 330–333.
- Gordon, N.; Colorni, A. Prymnesium parvum, an Ichthyotoxic Alga in an Ornamental Fish Farm in Southern Israel. Israeli J. Aquacult. Bamidgeh 2008, 60, 5–8.
- Rip, W.J.; Everards, K.; Houwers, A. Restoration of Botshol (The Netherlands) by reduction of external nutrient load: The effects on physico-chemical conditions, plankton and sessile diatoms. Hydrobiol. Bull. 1992, 25, 275–286.
- Johnsen, T.M.; Eikrem, W.; Olseng, C.D.; Tollefsen, K.E.; Bjerknes, V. Prymnesium parvum: The Norwegian Experience 1. JAWRA J. Am. Water Resour. Assoc. 2010, 46, 6–13.
- U.S. Geological Survey. Nonindigenous Aquatic Species Database. Available online: https://nas.er.usgs.gov/queries/collectioninfo.aspx?SpeciesID=3234 (accessed on 25 April 2023).
- Caron, D.A. Temporal and Geographic Progression of Prymnesium parvum (the ‘Golden Alga’) in the Southwestern United States; Department of Biologial Sciences, University of Southern California: Los Angeles, CA, USA, 2017.
