Along with the development of technology and increasing processing of raw materials used in various industries, there is a growing demand for research aimed at monitoring the ongoing changes and the general condition of the natural environment. They are caused by both natural processes over which humans have no direct influence and by the activities of society. Devices that allow to quickly and easily determine the content of ions in various types of environmental samples are ion-selective electrodes (ISEs), which are currently the most popular potentiometric sensors and have been the subject of research for many scientists around the world continuously for many years, thanks to their numerous advantages [
1,
2]. The enormity of possibilities offered by modifications of ion-selective electrodes, including the development of wearable sensors [
3,
4] and multi-sensor platforms [
5,
6,
7,
8] for simultaneous monitoring of ion concentrations in a continuous mode in the in situ environment, causes that research on new constructions of electrodes to achieve better and better analytical and electrical parameters is still being published [
9]. ISEs are currently used to determine the concentration of various types of ions (both inorganic and organic) in liquid samples in many areas of human life (agriculture [
10,
11], food industry [
11,
12,
13] and pharmaceutical industry [
14,
15], process control or clinical diagnostics [
16,
17,
18,
19]). ISEs can be used to determine the content of selected ions in natural water samples, both surface waters (rivers, lakes and seas) and groundwater, as well as in tap water and sewage [
20,
21,
22,
23].
2. Potentiometry and Ion-Selective Electrodes
Potentiometry is a highly selective and relatively cheap method that allows achieving low detection limits and a very wide dynamic range of sensors (up to eight orders of units) [
28,
29]. The principle of the method is to measure the electromotive force (EMF) of a cell made of two types of electrodes: a reference electrode whose potential has to necessarily be constant regardless of the composition and concentration of the sample, and an indicator (working) electrode whose potential changes depending on the activity of the main ion present in the sample solution to which the ion-selective membrane is sensitive [
30]. A potentiometric response is obtained, which is dependent on potential changes in real time [
31]. In most cases, no steps are necessary to prepare the sample for measurement. Moreover, the wide range of linearity of the method allows to avoid dilution or concentration of the sample solution, as is often necessary using ASA or HPLC methods. Most often (if necessary), it is limited to a small addition of ionic strength buffer and/or other substances to the sample solution, immediately before measurement and mixing. Sometimes it can also be necessary to ensure the correct pH of the environment when the pH of the test sample is not in the range, where the electrodes can work without interference. However, in most cases, this range is wide enough to allow the determination of ions directly in the collected sample. The great advantage of ISEs is the ability to test colored or muddy solutions, because both the color and the presence of solid particles do not interfere with the tests.
Among the various constructions of ISEs, a special place is held by electrodes without an internal electrolyte solution, the so-called ion-selective electrodes with solid contact (SCISEs). SCISEs are much more convenient during use, transport and storage, and they are easier to miniaturize and modify their shape and also more mechanically resistant. In addition, they can work in any position and in conditions of increased pressure and temperature, which results from the elimination of the internal solution present in classic electrodes, which acts as a link between the discharge electrode and the ion-selective membrane [
13]. For electrodes of this type, it is very important to properly select the material that will function as an ion–electron transducer, thus enabling the correct operation of the electrodes by ensuring appropriate stability and reproducibility of the potential. When developing a new type of SCISEs, there are two main ways to improve their analytical parameters. The first is the use of a new active substance which as a membrane component is responsible for the appropriate selectivity of the sensors towards the selected main ion (especially important in the study of complex samples containing interferences that may interfere with the proper measurement), and the second is the search for new electroactive materials that can be successfully used as solid contact. The use of solid contact is additionally aimed at obtaining a satisfactory stability of the sensor potential, which will allow doing measurements for a longer period of time (weeks, months), often without the need for frequent calibration [
9]. Electrodes used for this purpose should also be resistant to changes in measurement conditions (temperature, lighting and the presence of gases) [
32]. Due to the common trend towards miniaturization of devices (also due to much smaller amounts of materials used for their construction and compatibility with small volumes of samples necessary to perform measurements) and the desire to use them directly in the natural environment, it is necessary to obtain sensors characterized by sufficiently good values of analytical parameters and, at the same time, easy to use and mechanically resistant [
9]. Reliable measurement results can only be achieved with electrodes exhibiting a specific set of characteristics.
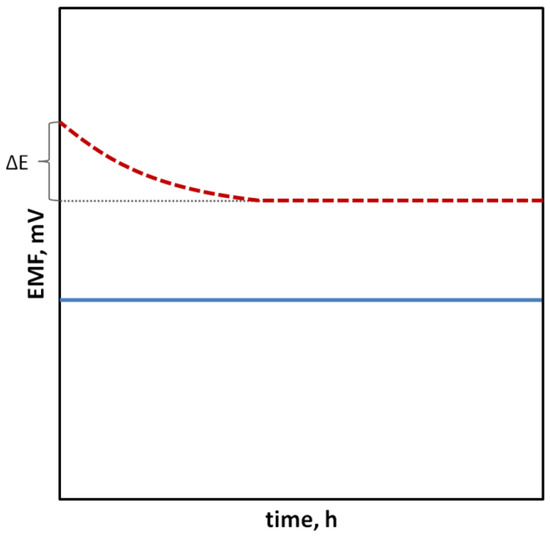
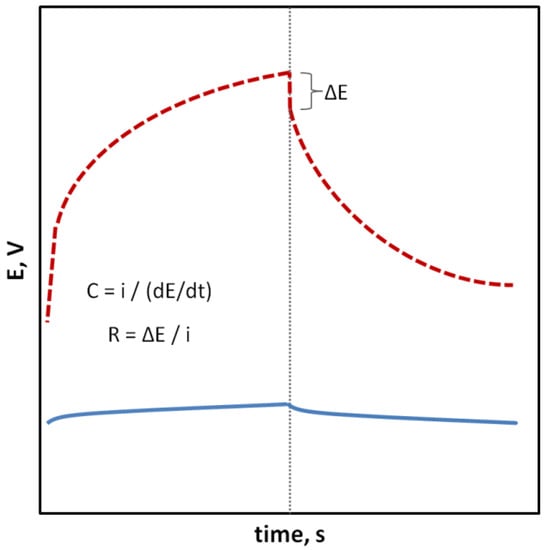
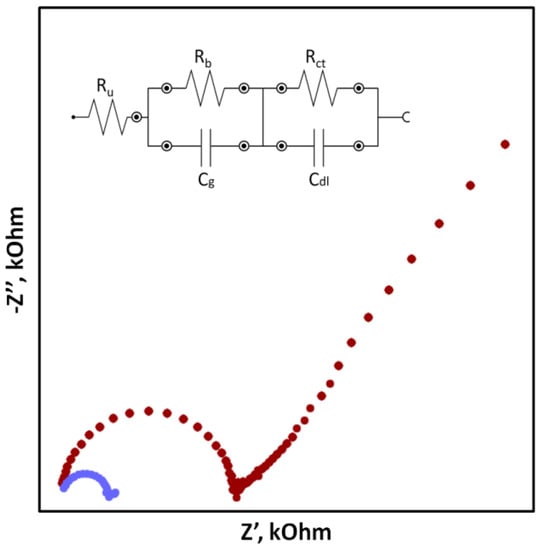
In the case of SCISEs, potential stability, both short-term and long-term, is particularly important. Long-term stability can be determined by systematically measuring the potential of the sensors at constant intervals over an extended period of time (e.g., days, weeks and months). This parameter is usually characterized as the change in potential E
0 over time. With regard to short-term stability, two approaches are possible. The first one consists of continuous measuring of the electrode potential in a relatively short time (1–3 h, that is the time needed to perform calibration and a series of measurements) in no-current conditions and determining the potential drift as ΔEMF/Δt (
Figure 1). The second faster way uses the chronopotentiometry method (CP) and the measurement of the changing electrode potential in time (for example, 60 s) after applying a short-term electrical impulse (the most often in nA) to the electrode. The potential drift, which is a measure of stability, is determined from a rectilinear section of the chronopotentiometric curve from the dE/dt relationship. Based on the determined potential drift, the capacitance of the electrodes can be determined according to the equation C = i/(dE/dt). The chronopotentiometry technique can also be used to determine the resistance of the electrode, which often changes as a result of adding a solid contact material (
Figure 2) [
33]. Another technique commonly used to assess the effectiveness of a solid contact material is electrochemical impedance spectroscopy (EIS), an advanced method for studying electrode processes. In the case of ISEs, this method allows the determination of the membrane resistance, electric capacitance and charge transfer resistance between the ion-sensitive membrane and the internal electronic conductor [
34,
35,
36]. These data are determined on the basis of the analysis of the impedance spectrum determined in a wide frequency range of 0.1 Hz–100 kHz by the semicircle method or by fitting an equivalent electrical circuit (
Figure 3).
Figure 1. Change in the electrode potential immersed in the solution of the main ion in time, indicating electrode with more stable (⸺) and less stable (- - -) potential.
Figure 2. Chronopotentiogram representing an electrode with a stable (⸺) and less stable (- - -) potential; formulas for determining the electric capacitance (C) and total resistance (R), where potential drift (dE/dt), ΔE—potential jump as a result of changing the direction of the electric current (i).
Figure 3. Exaplary impedance spectra for coated disc electrode without solid contact (red dots) and solid contact electrode (blue dots) with an electrical circuit (where Ruuncompensated series resistance; Rb-bulk resistance; Cg-geometric capacitance; Rct-charge transfer resistance; Cdllow-frequency layer capacitance).
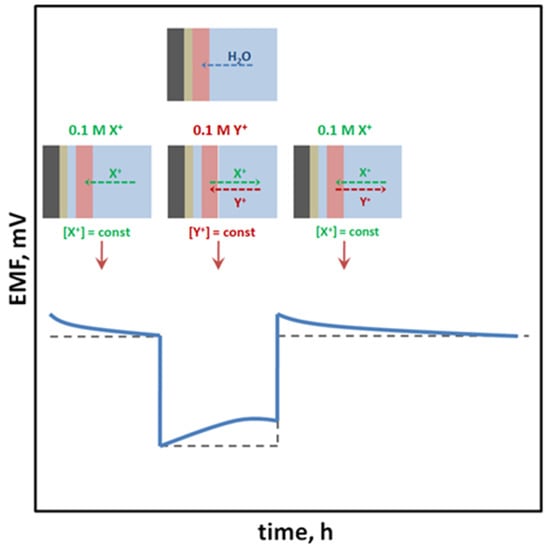
SCISEs are tested for the formation of a water layer, which is formed as a result of water uptake and transport through the ion-selective membrane material [
1]. The composition of the water layer may change during the modification of the sample solution composition in which the electrode is immersed, due to the penetration of interfering ions inside the layer, which is the reason for the drift of the measured potential [
37]. This is a particularly undesirable process; therefore, water layer tests are performed (usually in accordance with the procedure proposed by Fibbioli et al. [
37]) consisting in the use of high-concentration solutions of the main ion and interfering ion, and the observation of the potential drift caused by the change of solutions (in the order: main ion → interfering ion → main ion). Electrodes without a tendency to form a water layer maintain a constant potential after reaching a certain value after immersing the sensor in the interfering ion solution and quickly return to the initial potential value previously obtained in the solution of the main ion to which the membrane is sensitive (
Figure 4).
Figure 4. Results of the water layer test obtained for (⸺) electrodes with a tendency to form an undesirable water layer and (- - -) electrodes without this tendency.