Nanotechnology pertains to the employment of nanoparticles and furnishes the potential to fabricate novel materials and products possessing improved quality. The nanomaterials may be used as; nanosensors, nanocides, nanofertilizers, nanobarcodes, and nano-remediators, which play a significant role in modern agricultural practices. However, the physical and chemical processes of nanoparticle production is neither economical nor environmentally sustainable. Therefore, the need for green or biogenic nanoparticles obtained from plants, bacteria, fungi or their metabolites has emerged as novel, sustainable, economical, biocompatible, and eco-friendly technology.
- green nanotechnology
- green nanoparticles
- biogenic nanoparticles
- agriculture
1. Introduction
2. Biogenic Nanoparticles and Their Synthesis
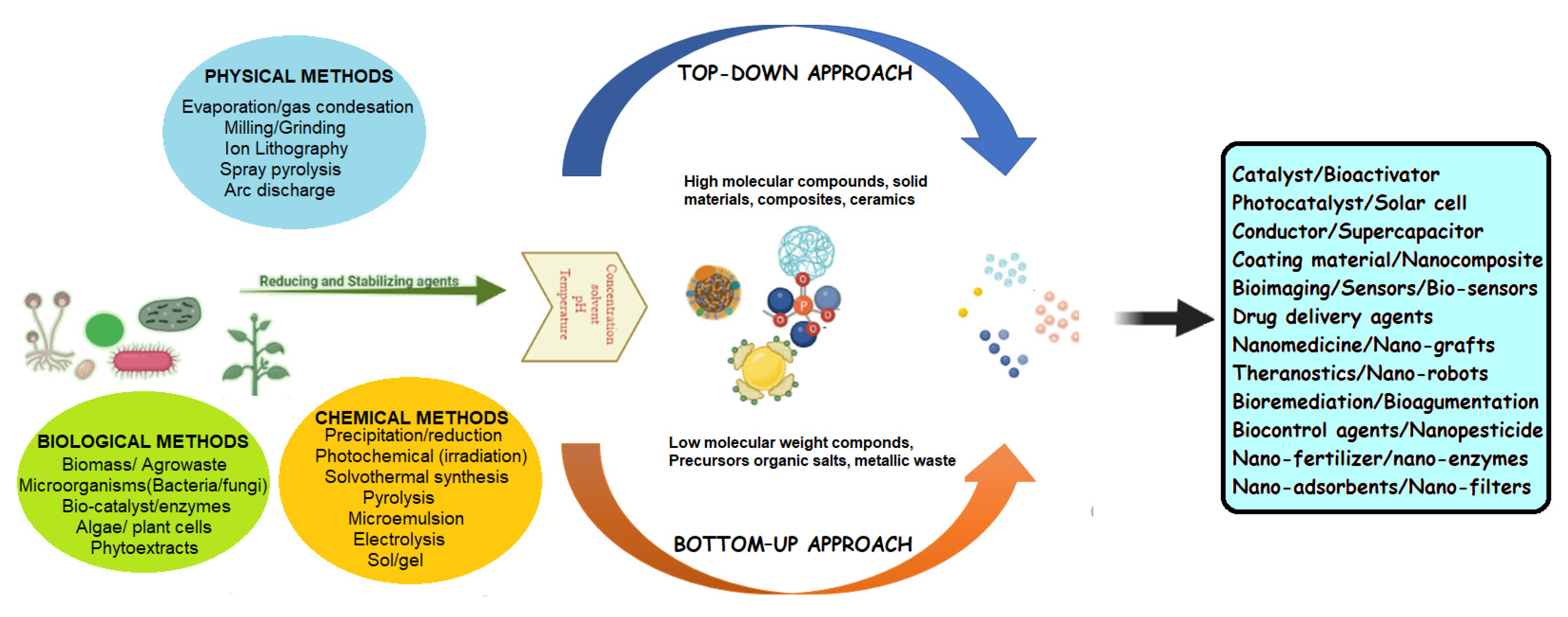
2.1. Conventional Synthesis Method
2.2. Green Synthesis Method
2.3. Nanoparticle Synthesis by Bacteria
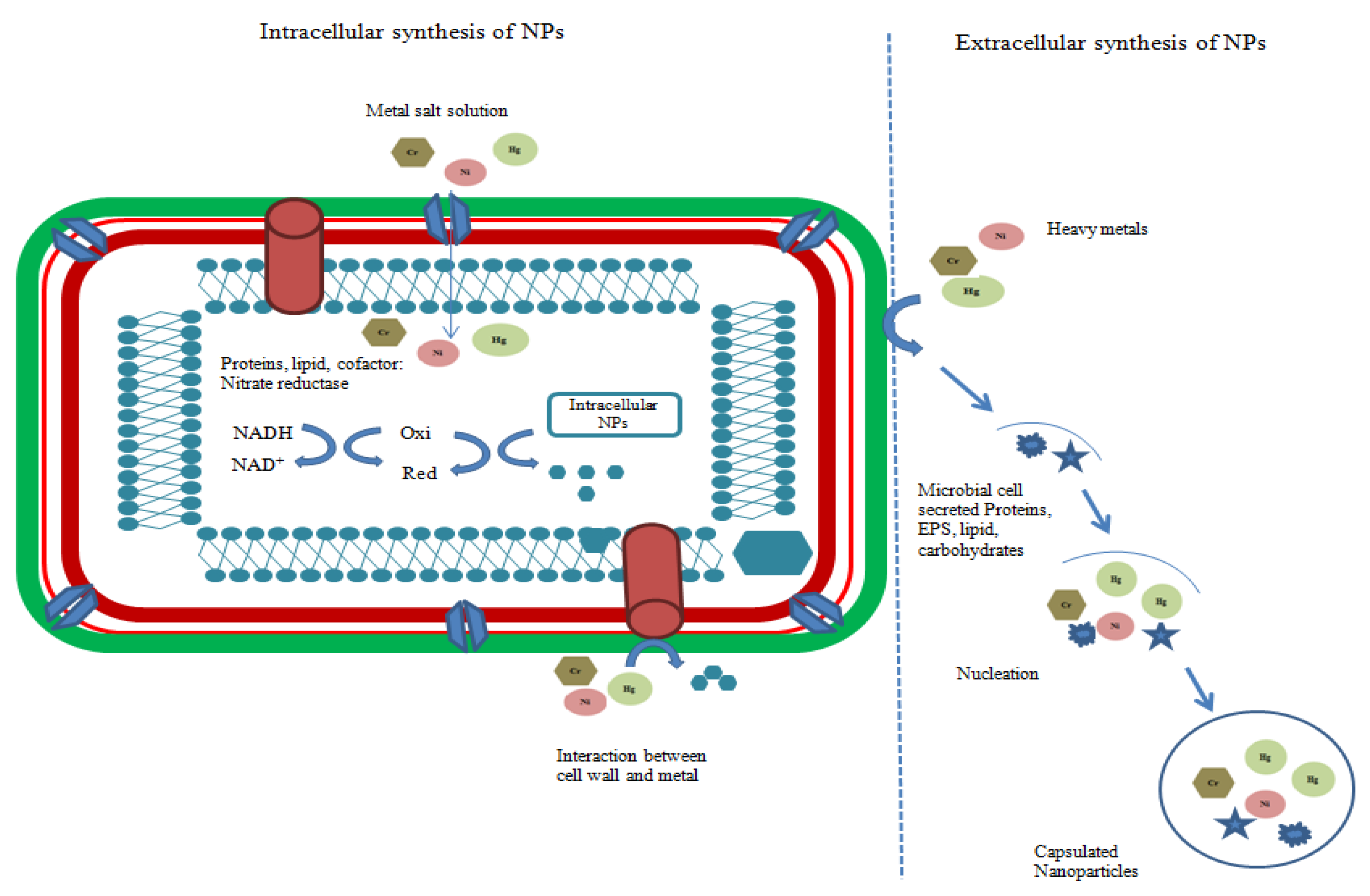
2.4. Nanoparticle Synthesis by Fungi
2.5. Nanoparticle Synthesis by Algae
2.6. Nanoparticle Synthesis by Plants
3. Characterization of Biogenic Nanoparticles
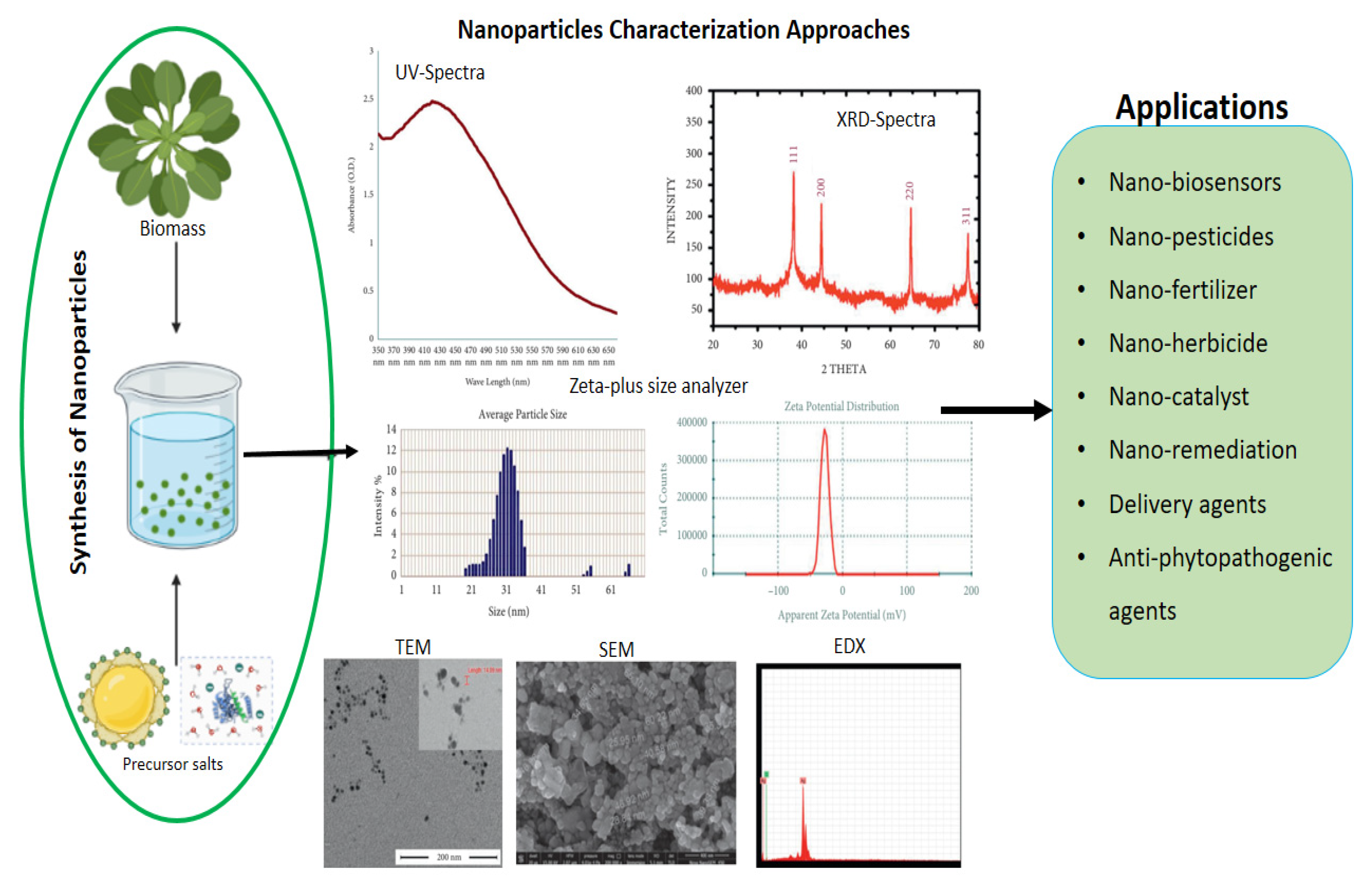
4. Application of Biogenic Nanoparticles in Sustainable Agricultural Practices
4.1. Application of Biogenic NPs as Nanofertilizers
4.2. Application of Biogenic NPs as Nanopesticides
4.3. Applications of NPs in Abiotic Stress Mangament
4.4. Applications of NPs as Nanobiosensors
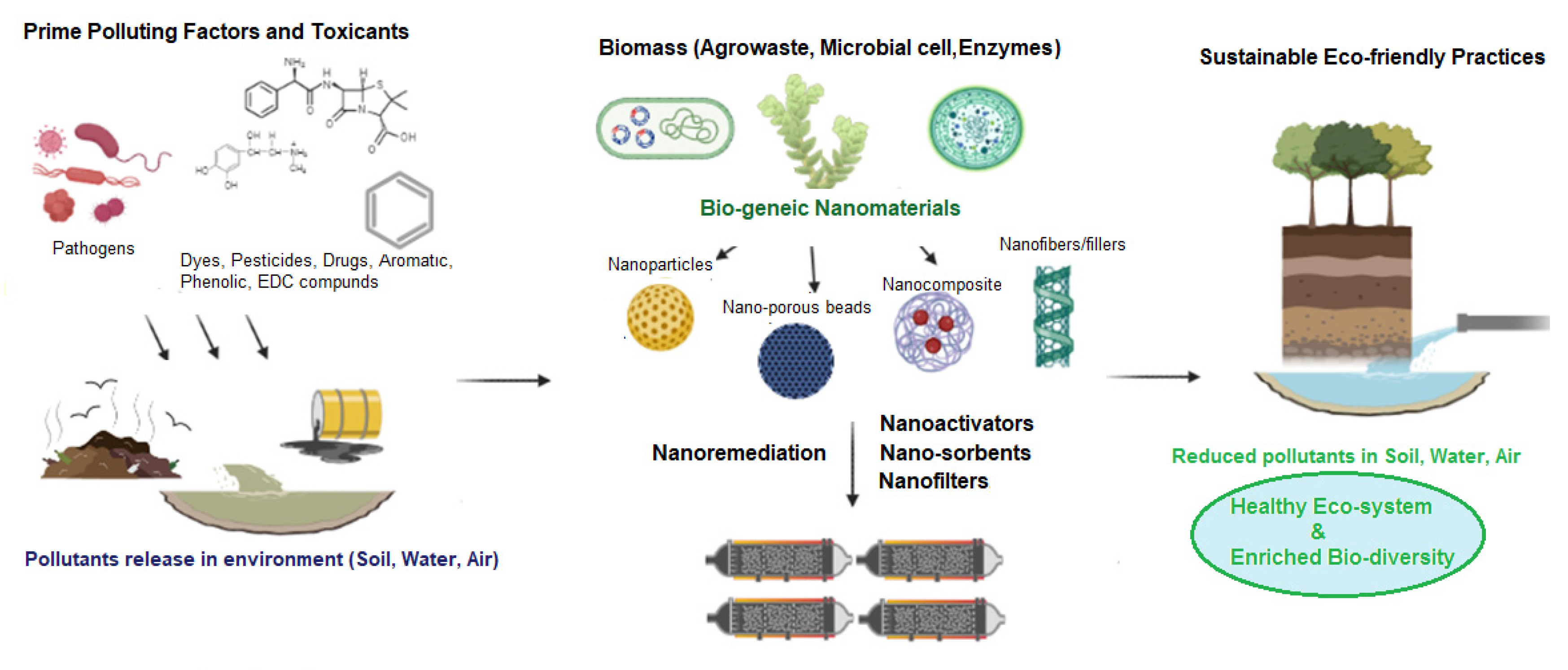
4.5. Application of Biogenic Nanoparticles in Bioremediation
This entry is adapted from the peer-reviewed paper 10.3390/agriculture13030668
References
- Ahmed, T.; Noman, M.; Manzoor, N.; Shahid, M.; Abdullah, M.; Ali, L.; Wang, G.; Hashem, A.; Al-Arjani, A.F.; Alqarawi, A.A.; et al. Nanoparticle-based amelioration of drought stress and cadmium toxicity in rice via triggering the stress responsive genetic mechanisms and nutrient acquisition. Ecotoxicol. Environ. Saf. 2021, 209, 111829.
- Khan, M.I.R.; Palakolanu, S.R.; Chopra, P.; Rajurkarm, A.B.; Gupta, R.; Iqbal, N.; Maheshwari, C. Improving drought tolerance in rice: Ensuring food security through multi- dimensional approaches. Physiol. Plant. 2021, 172, 645–668.
- Chaudhary, P.; Chaudhary, A.; Bhatt, P.; Kumar, G.; Khatoon, H.; Rani, A.; Kumar, S.; Sharma, A. Assessment of Soil Health Indicators Under the Influence of Nanocompounds and Bacillus spp. in Field Condition. Front. Environ. Sci. 2022, 9, 769871.
- Nejatzadeh, F. Effect of silver nanoparticles on salt tolerance of Satureja hortensis L. during in vitro and in vivo germination tests. Heliyon 2021, 7, e05981.
- Abdelkhalek, A.; El-Gendi, H.; Alotibi, F.O.; Al-Askar, A.A.; Elbeaino, T.; Behiry, S.I.; Abd-Elsalam, K.A.; Moawad, H. Ocimum basilicum-mediated synthesis of silver nanoparticles induces innate immune responses against cucumber mosaic virus in squash. Plants 2022, 11, 2707.
- Patil, S.; Chandrasekaran, R. Biogenic nanoparticles: A comprehensive perspective in synthesis, characterization, application and its challenges. J. Genet. Eng. Biotechnol. 2020, 18, 67.
- Mughal, B.; Zaidi, S.Z.J.; Zhang, X.; Hassan, S.U. Biogenic Nanoparticles Synthesis, Characterisation and Applications. Appl. Sci. 2021, 11, 2598.
- Sengani, M.; Chakraborty, S.; Balaji, M.P.; Govindasamy, R.; Alahmadi, T.A.; Obaid, S.A.; Karuppusamy, I.; Chi, N.T.L.; Brindhadevi, K.; Devi, R.V. Anti-diabetic efficacy and selective inhibition of methyl glyoxal, intervention with biogenic Zinc oxide nanoparticle. Environ. Res. 2023, 216, 114475.
- Jaiswal, K.K.; Banerjee, I.; Dutta, S.; Verma, R.; Gunti, L.; Awasthi, S.; Bhushan, M.; Kumar, V.; Alajmi, M.F.; Hussain, A. Microwave-assisted polycrystalline Ag/AgO/AgCl nanocomposites synthesis using banana corm (rhizome of Musa sp.) extract: Characterization and antimicrobial studies. J. Ind. Eng. Chem. 2022, 107, 145–154.
- Kavitha, G.; Kumar, J.V.; Pavithra, S.; Komal, M.; Nivetha, M.S.; Kayalvizhi, R.; Abirami, N. Biogenic synthesis of argentum nanocomposites for visible light photocatalyst of dye degradation. Chem. Phys. Lett. 2022, 809, 140159.
- Chakraborty, S.; Singh, A.; Roychoudhury, A. Biogenic nanoparticles and generation of abiotic stress-resilient plants: A new approach for sustainable agriculture. Plant Stress 2022, 6, 100117.
- Mittal, D.; Kumar, A.; Balasubramaniam, B.; Thakur, R.; Siwal, S.S.; Saini, R.V.; Gupta, R.K.; Saini, A.K. Synthesis of Biogenic silver nanoparticles using plant growth promoting bacteria: Potential use as biocontrol agent against phytopathogens. Biomat. Polym. Horiz. 2022, 1, 22–31.
- Rai, M.; Ingle, A.P.; Trzcinska-Wencel, J.; Wypij, M.; Bonde, S.; Yadav, A.; Kratošová, G.; Golinska, P. Biogenic Silver Nanoparticles: What We Know and What Do We Need to Know? Nanomaterials 2021, 11, 2901.
- Revati, K.; Pandey, B.D. Microbial synthesis of iron-based nanomaterials—A review. Bull. Mater. Sci. 2011, 34, 191–198.
- Durán, N.; Marcato, P.D.; Durán, M.; Yadav, A.; Gade, A.; Rai, M. Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants. Appl. Microbiol. Biotechnol. 2011, 90, 1609–1624.
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566.
- Nangia, Y.; Wangoo, N.; Goyal, N.; Sharma, S.; Wu, J.S.; Dravid, V.; Shekhawat, G.S.; Suri, C.R. Facile biosynthesis of phosphate capped gold nanoparticles by a bacterial isolate Stenotrophomonas maltophilia. Appl. Phys. Lett. 2009, 94, 233901.
- Gahlawat, G.; Choudhary, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967.
- Mukherjee, P.; Roy, M.; Mandal, B.P.; Dey, G.K.; Mukherjee, P.K.; Ghatak, J.; Tyagi, A.K.; Kale, S.P. Green synthesis of highly stabilized nanocrystalline silver particles by a nonpathogenic and agriculturally important fungus T. asperellum. Nanotechnology 2008, 19, 103–110.
- Jalal, M.; Ansari, M.A.; Alzohairy, M.A.; Ali, S.G.; Khan, H.M.; Almatroudi, A.; Raees, K. Biosynthesis of silver nanoparticles from oropharyngeal Candida glabrata Isolates and their antimicrobial activity against clinical strains of bacteria and fungi. Nanomaterials 2018, 8, 586.
- Kalpana, V.N.; Kataru, B.A.S.; Sravani, N.; Vigneshwari, T.; Panneerselvam, A.; Rajeswari, V.D. Biosynthesis of zinc oxide nanoparticles using ulture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. Open Nanomat. 2018, 3, 48–55.
- Annamalai, J.; Nallamuthu, T. Green synthesis of silver nanoparticles: Characterization and determination of antibacterial potency. Appl. Nanosci. 2016, 6, 259–265.
- Choudhary, M.K.; Kataria, J.; Sharma, S. Evaluation of the kinetic and catalytic properties of biogenically synthesized silver nanoparticles. J. Clean. Prod. 2018, 198, 882–890.
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262.
- Tombuloglu, H.; Slimani, Y.; Tombuloglu, G.; Alshammari, T.; Almessiere, M.; Korkmaz, A.D.; Baykal, A.; Samia, A.C.S. Engineered magnetic nanoparticles enhance chlorophyll content and growth of barley through the induction of photosystem genes. Environ. Sci. Pollut. Res. 2020, 27, 3431–3432.
- Raj, S.; Trivedi, R.; Soni, V. Biogenic Synthesis of Silver Nanoparticles, Characterization and Their Applications—A Review. Surfaces 2022, 5, 67–90.
- Tombuloglu, H.; Slimani, Y.; Alshammari, T.; Tombuloglu, G.; Almessiere, M.; Baykal, A.; Ercan, I.; Ozcelik, S.; Demirci, T. Magnetic Behavior and Nutrient Content Analyses of Barley (Hordeum vulgare L.) Tissues upon CoNd0.2Fe1.8O4 Magnetic Nanoparticle Treatment. J. Soil Sci. Plant Nutr. 2020, 20, 357–366.
- Tombuloglu, H.; Anıl, I.; Akhtar, S.; Turumtay, H.; Sabit, H.; Slimani, Y.; Almessiere, M.; Baykal, A. Iron oxide nanoparticles translocate in pumpkin and alter the phloem sap metabolites related to oil metabolism. Sci. Hortic. 2020, 265, 109223.
- Nguyen, T.M.T.; Huynh, T.T.T.; Dang, C.H.; Mai, D.T.; Nguyen, T.T.N.; Nguyen, D.T. Novel biogenic silver nanoparticles used for antibacterial effect and catalytic degradation of contaminants. Res. Chem. Intermed. 2020, 46, 1975–2090.
- Purohit, A.; Sharma, R.; Ramakrishnan, R.S.; Sharma, S.; Kumar, A.; Jain, D. Biogenic synthesis of Silver Nanoparticles (AgNPs) Using Aqueous Leaf Extract of Buchanania lanzan Spreng and Evaluation of Their Antifungal Activity against Phytopathogenic Fungi. Bioinorg. Chem. Appl. 2022, 2022, 6825150.
- Agri, U.; Chaudhary, P.; Sharma, A.; Kukreti, B. Physiological response of maize plants and its rhizospheric microbiome under the influence of potential bioinoculants and nanochitosan. Plant Soil. 2022, 474, 451–468.
- Avila-Quezada, G.D.; Ingle, A.P.; Golińska, P.; Rai, M. Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks. Nanotechnol. Rev. 2022, 11, 2123–2140.
- Shinde, S.; Paralikar, P.; Ingle, A.P.; Rai, M. Promotion of seed germination and seedling growth of Zea mays by magnesium hydroxide nanoparticles synthesized by the filtrate from Aspergillus niger. Arab. J. Chem. 2020, 13, 3172–3182.
- Salih, A.M.; Qahtan, A.A.; Al-Qurainy, F.; Al-Munqedhi, B.M. Impact of Biogenic Ag-Containing Nanoparticles on Germination Rate, Growth, Physiological, Biochemical Parameters, and Antioxidants System of Tomato (Solanum tuberosum L.) In Vitro. Processes 2022, 10, 825.
- Fouda, M.M.; Abdelsalam, N.R.; El-Naggar, M.E.; Zaitoun, A.F.; Salim, B.M.; Bin-Jumah, M.; Allam, A.; Abo-Marzoka, S.A.; Kandil, E.E. Impact of high throughput green synthesized silver nanoparticles on agronomic traits of onion. Int. J. Biol. Macromol. 2020, 149, 1304–1317.
- Krishnaraj, C.; Jagan, E.G.; Ramachandran, R.; Abirami, S.M.; Mohan, N.; Kalaichelvan, P.T. Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Proc. Biochem. 2012, 47, 51–658.
- Shaik, A.M.; David, R.M.; Reddy, D.R.S. Green synthesis of zinc oxide nanoparticles using aqueous root extract of Sphagneticola trilobata Lin and investigate its role in toxic metal removal, sowing germination and fostering of plant growth. Inorg. Nano-Metal Chem. 2020, 50, 569–579.
- Hassan, S.E.; Fouda, A.; Radwan, A.A.; Salem, S.S.; Barghoth, M.G.; Awad, M.A.; Abdo, A.M.; El-Gamal, M.S. Endophytic actinomycetes Streptomyces spp. mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. J. Biol. Inorg. Chem. 2019, 24, 377–393.
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28.
- Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-Álvarez, J.; Vega-Fernández, L.; Montes de Oca-Vásquez, G.; Vega-Baudrit, J.R. Green synthesis of gold and silver nanoparticles from plant extracts and their possible applications as antimicrobial agents in the agricultural area. Nanomaterials 2020, 10, 1763.
- Devatha, C.P.; Jagadeesh, K.; Patil, M. Effect of Green synthesized iron nanoparticles by Azardirachta indica in different proportions on antibacterial activity. Environ. Nanotech. Monit. Manag. 2018, 9, 85–94.
- González-Merino, A.M.; Hernández-Juárez, A.; Betancourt-Galindo, R.; Ochoa-Fuentes, Y.M.; Valdez-Aguilar, L.A.; Limón-Corona, M.L. Antifungal activity of zinc oxide nanoparticles in Fusarium oxysporum–Solanum lycopersicum pathosystem under controlled conditions. J. Phytopat. 2021, 169, 533–544.
- Sreelakshmi, B.; Induja, S.; Adarsh, P.; Rahul, H.; Arya, S.; Aswana, S.; Haripriya, R.; Aswathy, B.; Manoj, P.; Vishnudasan, D. Drought stress amelioration in plants using green synthesised iron oxide nanoparticles. Mater. Today Proc. 2020, 41, 723–727.
- Zafar, S.; Hasnain, Z.; Aslam, N.; Mumtaz, S.; Jaafar, H.Z.; Wahab, P.E.M.; Qayum, M.; Ormenisan, A.N. Impact of Zn nanoparticles synthesized via green and chemical approach on okra (Abelmoschus esculentus L.) growth under salt stress. Sustainability 2021, 13, 3694.
- Kalyani, N.; Goel, S.; Jaiswal, S. On-site sensing of pesticides using point-of-care biosensors: A review. Environ. Chem. Lett. 2021, 19, 345–354.
- Thakur, M.; Wang, B.; Madan, L.; Verma, L. Development and applications of nanobiosensors for sustainable agricultural and food industries: Recent developments, challenges and perspectives. Environ. Technol. Inn. 2022, 26, 102371.
- James, A.; Franco, F.; Merca, E.M.; Rodriguez, S.; Johny, F.; Balidon-Veronica, P.; Divina, M.; Evangelyn, A.; Alocilja, C.; Fernando, L.M. DNA based electrochemical nanobiosensor for the detection of Phytophthora palmivora causing black pod rot in cacao (Theobroma cacao L.) pods. Physiol. Mol. Plant Pathol. 2019, 107, 14–20.
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23.
- Giraldo, J.P.; Wu, H.; Newkirk, G.M.; Kruss, S. Nanobiotechnology approaches for engineering smart plant sensors. Nat. Nanotechnol. 2019, 14, 541–553.
- Malik, S.; Dhasmana, A.; Preetam, S.; Mishra, Y.K.; Chaudhary, V.; Bera, S.P.; Ranjan, A.; Bora, J.; Kaushik, A.; Minkina, T.; et al. Exploring Microbial-Based Green Nanobiotechnology for Wastewater Remediation: A Sustainable Strategy. Nanomaterials 2022, 12, 4187.
