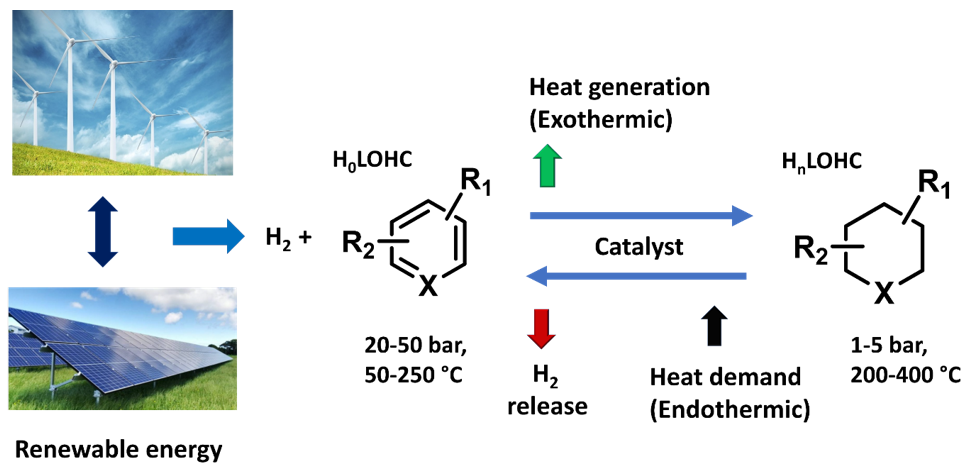
Molecular liquid that can accommodate and release hydrogen molecules via chemical hydrogenation and dehydrogenation.
- LOHC
- Hydrogen storage
- Liquid
- Hydrogenation
1. Introduction
Energy is crucial for development of the modern world. So far, most energy requirements are fulfilled by fossil-based fuels. Indeed, rapid industrialization and advanced technologies affect the fuel economy, and in other direction, the vast consumption of fossil energies causes anthropogenic global warming, which negatively impacts the environment and human health. Moreover, our giant usage engenders the depletion of fossil-based resources and faces severe shortage in the near future. In fact, renewable energy sources, such as solar and wind, have attracted enormous attention [1]. However, these resources are not yet stable and fluctuate depending on the season. Alternatively, batteries reinforce global electric transportation [2]; however, this technology presents limitations, such as slow charging rates, scarcity of Li and Co with large-scale utility, and may be costly [3]. Hence, alternative energy sources must be sought to conquer the current energy demands. In this context, hydrogen is a clean and efficient energy carrier and can be employed as a carbon-emission free fuel for a variety of appliances, such as fuel-cell vehicles, stationary and portable electronics, etc. [4][5].
Hydrogen (H2) is the lightest element in the periodic table; it is a colorless, odorless, and tasteless gas with a low volumetric density of 0.08988 g/L at 101,325 Pa [6]. The idea of using hydrogen as an energy source and carrier was presented several decades ago. In 1971, Jones postulated that the use of liquid hydrogen must be seriously considered as a logical replacement for hydrocarbon fuels in the 21st century [7]. Substantially, Winsche et al. [8], Momirlan et al. [9], and Bockris [10] have been highlighted the hydrogen roles and its benefits in the future hydrogen fuel economy. Though hydrogen is anticipated to be a clean and efficient energy carrier, production is highly challenging. In this respect, there are different varieties of primary energy sources, as well as various efficient technologies that have been developed for the production of hydrogen. Mostly, non-renewable resources such as natural gas and coal are displaying similar participation in H2 production. Nevertheless, renewable and sustainable energy (RSE) sources have shown significant attention for long-term production of clean hydrogen, for example, the splitting of water into hydrogen (H2) and oxygen (O2) by electrolysis process [11] and the generating electricity by wind energy, which can be utilized in water electrolysis. The ubiquitous RSE source, solar energy, also produces hydrogen by employing sunlight as an energy source in water splitting process. Furthermore, biomass energy can generate hydrogen by biological and thermochemical processes [12][13]. Despite extensive research toward the production of clean H2, safe and cost-effective storage and transportation of hydrogen is a major task in the development of a hydrogen economy. To date, many physical and chemical hydrogen storage techniques have been extensively investigated. In physical storage methods, high pressure compression in cylinders (up to 7 × 104 kPa) and liquefaction of hydrogen are commercially used. However, hydrogen compression in capable cylinders should withstand high pressures ((2–7) × 104 kPa) and required expensive composite materials (aluminum, steel, or thermoplastic-lined carbon fibers) for making these storage tanks [14]. Then, liquifying hydrogen (−253 °C) in cryogenic tanks also requires expensive, multi-storage cooling protocols [15]. In contrast, chemical storage techniques comprised of metal hydrides and their alloys [16][17] (e.g., MgH2, LaNi5H6, NaAlH4), store hydrogen via chemisorption and physisorption of hydrogen by porous materials [18][19][20] (e.g., activated carbon, graphene, carbon nanotubes, and metal-organic frameworks), in which these are more favorable storage methods. Nevertheless, metal hydrides and associated complexes face severe issues, such as lower gravimetric hydrogen capacities (<5.5 wt%; In 2010, the Department of Energy (DOE) target hydrogen capacity was 6 wt% for entire systems including tanks, regulators, valves, etc., and in 2017, the DOE designated targets of 5.5 wt%, 40 g L−1, for mobile applications [21].), limited reversibility at optimal pressure-temperature regions, and instability of storage materials. Furthermore, physisorption of solid materials requires extremely low temperatures (−196 °C). Given this, research efforts have drawn attention toward the development of alternative hydrogen storage compounds, known as liquid-organic hydrogen carriers (LOHCs).
By definition, LOHCs are organic compounds which exist as liquids or low melting point solids under ambient storage conditions. Indeed, LOHC systems are potentially safe and relatively cheap storage materials. These systems can have a pair of hydrogen-rich (H2+) and hydrogen-lean (H2−) molecules. In this system, hydrogen is stored by H2− molecules through catalytic hydrogenation (exothermic), and hydrogen is released by catalytic dehydrogenation (endothermic) reactions of H2+ molecules at optimal temperature and pressure conditions (Scheme 1). Notably, the high gravimetric and volumetric storage of hydrogen in small organic molecules have shown significant promise due to their numerous advantages, such as easy and clean energy storage without any concept-induced leakages, compatibility with present transport and refueling infrastructures, as well as operation under ambient conditions (pressure or temperature). To date, several efficient LOHC compounds have been developed. However, research efforts toward their development and practical usage is still in infancy. Thus, based on reported literature [22][23][24], a LOHC system should meet the following characteristic properties in order to employ as good candidate for practical applications.
- It should be non-toxic and safe, with an acceptable eco-toxicology profile during transportation and usage.
- To avoid the need for solid-based fuel infrastructure and external addition of solvents, LOHC systems should have low melting points with favorable values <−30 °C.
- The boiling point of the LOHC system should be high (>300 °C) to simplify the purification of hydrogen and require low dynamic viscosity for easy pumping.
- Reasonably high volumetric (>56 kg/m3) and gravimetric storage capacities (>6 wt%) are required.
- To attain the stability of LOHC molecules and achieve low dehydrogenation temperatures (<200 °C at 100 kPa H2 pressure), the desired hydrogen binding enthalpy should be in the range of 40–70 kJ/mol H2, based on Wild et al. [25] and 42–54 kJ/mol H2 per Cooper et al. [26].
- The system should be able to liberate sufficiently pure H2 while producing very selective hydrogenated and dehydrogenated products over long-life cycles, as well as avoiding alternative decomposition pathways.
- It should be compatible with existing fuel infrastructure and have low production costs.
Therefore, the above-mentioned characteristic properties pave the way for novel discoveries toward the development of efficient LOHC candidates in the near future. However, none of the known LOHCs, such as naphthalene, N-ethyl carbazole (NEC), etc., have achieved these properties to the full extent.
Scheme 1. Schematic representation of the LOHC concept.
2. Brief History of LOHCs
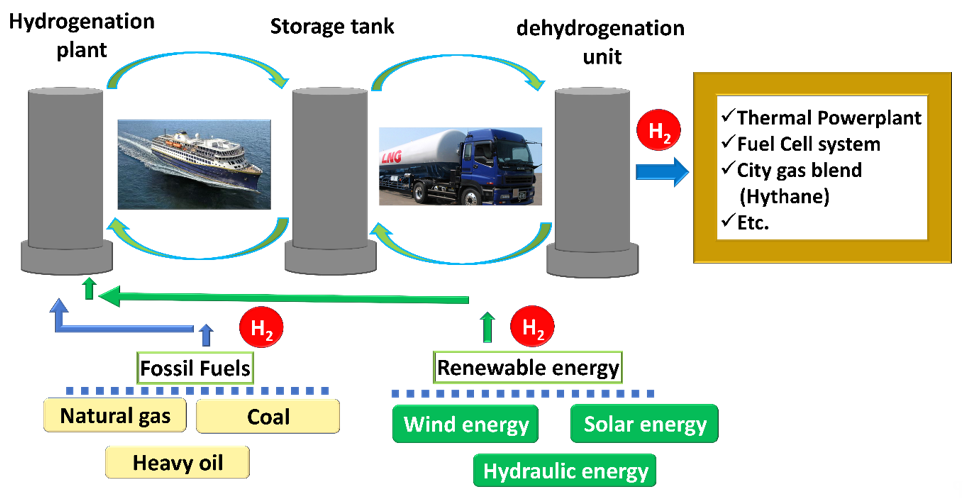
Research studies toward hydrogen storage in LOHCs via hydrogenation/dehydrogenation processes first took place in the early 1980s [27]. Based on the (de)hydrogenation processes, the most predominant task was pointed out as toluene/methylcyclohexane (MCH) system [28]. Following the MCH system research, numerous LOHC concepts have been assessed based on hydrogenation and dehydrogenation criteria for hydrogen storage. In the early 2000s, the basic concept of a cyclohexane/benzene LOHC system was investigated by Japanese researchers and they examined similar systems in more detail [29][30]. Notably, NEC was proposed as a LOHC candidate by Pez et al. in 2005 [31], and then in-depth research of this carrier material has been continued by various groups [32][33][34]. The latter, different research groups have also shown significant interest in alternative azaborine carrier materials [35][36][37]. In 2008, Crabtree suggested that N-containing heterocyclics are more advantageous LOHC materials, in terms of ease of H2 release, safe storage, low vapor pressure, better biodegradability, and simple heat management [38]. Muller et al. proposed that nitrogen-containing aromatic compounds are well-suited for better hydrogen storage based on thermodynamic evaluation [39], emphasized by their enthalpy changes during hydrogenation. Based on the LOHC concept, a mile stone was reached by the Chiyoda Corporation (Japan; Scheme 2) [40]. This company completed a pilot-plant facility for large-scale hydrogenation and dehydrogenation of LOHC materials in 2018. Additionally, numerous potential LOHC candidates were proposed and exploited in practical applications such as decentralized energy storage network [41], combined heat and power (CHP) systems [42]. Eypasch et al. demonstrated theoretical assumption of energy supply based on LOHC for industrial production plants [43]. Very recently, Niermann et al. indicated that LOHCs are technologically efficient and economically promising safe transportation and storage materials [44].
Scheme 2. The demonstration plan for large-scale hydrogen storage and transportation, as proposed by Chiyoda Corporation (Japan).
In this context, a wide range of potential LOHCs have been proposed in the literature and are highlighted based on the thermodynamic properties [38][39]. Efficient LOHCs have been proposed, and important insights into catalytic processes have been gleaned from theoretical approaches such as density functional theory (DFT) and ab initio-DFT calculations [22][45]. Further, contributions regarding fundamental catalytic aspects for hydrogenation and dehydrogenation of LOHCs have also been discussed [46].
3. Critical Issues in Developing LOHC Media
According to reported literature, we briefly discuss a few important LOHC characteristic properties and key aspects of LOHC catalytic systems as well as numerous other factors more generally for practical implementation of LOHCs.
3.1. Hydrogenation/Dehydrogenation
Usually, reversible hydrogenation and dehydrogenation at ambient temperature conditions is the primary requirement for hydrogen storage LOHC candidates. Thermodynamically, the feasibility of these reactions is strongly influenced by the thermodynamic reaction enthalpies. In particular, aromatic hydrogenation reactions are highly exothermic and thermodynamically favorable (e.g., aromatic benzene ring enthalpy is ΔRh = −68.73 kJ/mol H2, but the released energy not typically used. On the other hand, dehydrogenation is endothermic and requires high heat demand which is in the range of 64–69 kJ/mol H2, and as a result, this reaction is unfavorable both kinetically and thermodynamically (e.g., cyclohexane/benzene and MCH/toluene pairs) [47][48]. In contrast, liquid hydrogen carriers require low heat management though it needs an active catalyst in the dehydrogenation process. Considering these thermodynamic difficulties, Pez and coworkers for the first time suggested in their patent, the use of N-heterocyclics (e.g., NEC) decreases endothermicity and enhance hydrogen release as compared with alicyclics at relatively lower temperatures [31]. In addition, Crabtree and coworkers’ systematic computational studies on structural factors generalized the substitution of nitrogen atoms in five- and six-membered rings how judiciously achieving the lower H2 release temperatures [49]. Based on Muller et al.’s contribution, the facile reaction enthalpy for an ideal LOHC candidate is about 40 kJ/mol H2, and they suggested nitrogen-substituted aromatic compounds could reach this requirement easily, despite non-aromatic compounds being barely suitable [39]. Hence, these reports encourage the nitrogen-containing compounds are the focus of interest in developing novel LOHC systems.
3.2. Reaction Catalysts for LOHC
Another crucial aspect in LOHC development is selection of the proper catalytic system for hydrogenation and dehydrogenation reactions. As discussed in Section 3.1, chemical storage of hydrogen in liquid carriers is attained exothermically during hydrogenation process whereas endothermic liberation of hydrogen is observed during dehydrogenation process, moreover, this endothermicity is a major drawback due to the requirement of high heat demand. Though thermodynamic evaluation is the one of the concerns in designing the LOHCs, an active catalytic system with a high to moderate loading of precious metal catalysts (e.g., Pd, Pt, Rh, and Ru) can actually achieve acceptable dehydrogenation kinetics at low temperatures (≤150 °C). In particular, numerous homogenous catalysts [50][51][52] have been developed for this purpose; however, the achieved stability, recycling, and practical advantages in large-scale applications have more precisely promoted heterogeneous catalyst systems. Various commercial heterogenous catalysts with different supporting substances (e.g., Pd/C, Pt/Al2O3, and Pd-Pt/Al2O3) have attracted much attention for LOHC technology [46]. However, high catalyst efficiency is the major criteria in which metal loading, appropriate support selection, and structural properties (particle size, porosity, active surface area, etc.) are all important characteristics [53][54][55]. To this end, development and optimization of efficient catalyst systems obviously requires both experimental and theoretical approaches. Theoretical studies using DFT calculations can provide fundamental insight into catalytic activity and selectivity over different metal surfaces and experimental methods comprised of various spectroscopic techniques (typically X-ray photoelectron spectroscopy (XPS), temperature-programmed desorption (TPD), and infrared reflection absorption spectroscopy (IRAS), etc.) further support the field of LOHC dehydrogenation catalysis [46][56]. Combining these complementary approaches, the development of efficient catalytic systems is promising.
3.3. Features of LOHC Medium
3.3.1. Melting and Boiling Points
In 2017, the US DOE designated an organic hydrogen carrier target gravimetric storage capacity of 5.5 wt% H2 relative to the storage system including the carrier, tank, and dehydrogenation unit [21]. Predominantly, the carrier materials depend on two major concerns: storage density (typically 7.3 wt% for naphthalene) and existed temperature range in which the material remains a liquid. Despite the storage density requirement, carrier compounds can have low melting points (<−30 °C) [57]; otherwise need to use of additional solvents to dissolve it which hinders the targeted storage capacity. Usually, larger aromatic compounds, like naphthalene and anthracene have high storage capacities but solidify at room temperature. In this regard, destroying the symmetry by substituting alkyl chains (e.g., cyclohexane melts at 6.5 °C whereas MCH melts at −126.6 °C) is an alternative solution [58], though it decreases storage capacity. Besides, the boiling point (b.pt.) of carrier molecules should be as high as possible (>300 °C) which can minimize the vapor pressure during dehydrogenation and storage. In this case, larger aromatic compounds (e.g., b.pt. of anthracene is 342 °C) are more advantageous than smaller compounds (e.g., toluene b.pt. is 110.6 °C), except for the occurrence of their solid phase states. Therefore, these mentioned thermodynamic properties should be helpful in guiding future LOHC development.
3.3.2. Stability of LOHC Molecules
The consequent consideration in developing of ideal LOHCs is fall under the stability aspect of LOHC molecules. In LOHC technology, carrier molecule stability is strongly affected by hydrogenation and dehydrogenation temperatures under relevant catalytic medium. In particular, lower temperature conditions can avoid the decomposition pathways, thereby, yielding no side-product formation and allowing for long-term use. On the other hand, suitable catalysts with high activity can minimize the reaction temperatures. For example, carbazole-based derivatives undergo dealkylation which potentially leads to deterioration of LOHC materials at the required dehydrogenation temperatures. In this case, Amende et al. reported that control of particle size and structure-dependent effects of active metal catalysts on well-defined surfaces could benefit for long-term stability [56][59]. Thus, understanding both structural properties and theoretical studies on model catalysts and their activities can facilitate development of future LOHC molecules.
3.3.3. Toxicity and Biodegradability
Considering practical applicability, toxicity and biodegradability of LOHC materials must be evaluated. Owing to the increased benefits of LOHC technology, hazard assessment of molecules needs to be examined to minimize negative impacts on human health and the environment. As rated by the Toxicity Potential Indicator (TPI), values cover the range of “0” for non-toxic to “100” for extremely toxic [60]. Generally, toxicity assessment is more common for dehydrogenation counterparts than hydrogenated molecules [23]. For example, the safety data for the technical dibenzyl toluene mixture (Marlotherm SH; MSH) is described as low risk, and ecotoxicological problems are less than common diesel and are comparatively more favorable than NEC [61]. In addition, biodegradability is another key factor of a LOHC system. In this scenario, nitrogen-containing molecules typically have better biodegradability than alicyclic molecules, as reported by Crabtree [24]. Very recently, Markiewicz et al. reported hazard assessment of quinaldine, three-different alkyl carbazoles, benzene, and toluene based on mutagenicity, cytotoxicity, acute aquatic toxicity and biodegradability of each LOHC system [62]. Therefore, study on toxicity and biodegradability of LOHC molecules are imperative for developing LOHC systems.
In addition, there are several factors such as flashpoint, ignition temperature, density, viscosity, and surface tensions, that need be considered for the development of LOHC systems. Though these properties are not addressed to the full extent in the literature for all LOHC molecules, only few molecules are well-documented due to their utilization in mobile applications [61][63].
This entry is adapted from the peer-reviewed paper 10.3390/en13226040
References
- REN21. Renewables 2017. In Global Status Report; REN21: Montreal, QC, CA, 2017.
- Kim, S.J.; Cho, J.‐; Lee, K.‐Y.; Cho, B.W.; Chung, K.Y.; Kim, J.‐Y. MnOx–Carbon Black‐embedded LiFePO4(MnOx/C‐LFP) as a Cathode Material for High‐Power Li‐Ion Batteries. Bull. Korean Chem. Soc. 2019, 40, 317–323.
- Eberle, D.U.; von Helmolt, D.R. Sustainable transportation based on electric vehicle concepts: A brief overview. Energy Environ. Sci. 2010, 3, 689–699.
- Barreto, L.; Makihira, A.; Riahi, K. The hydrogen economy in the 21st century: A sustainable development scenario. J. Hydrog. Energy 2003, 28, 267–284.
- Sartbaeva, A.; Kuznetsov, V.L.; Wells, S.A.; Edwards, P.P. Hydrogen nexus in a sustainable energy future. Energy Environ. Sci. 2008, 1, 79–85.
- Saxena, R.C.; Seal, D.; Kumar, S.; Goyal, H.B. Thermo-chemical routes for hydrogen rich gas from biomass: A review. Renew Sust. Energ. Rev. 2008, 12, 1909–1927.
- Jones, L.W. Liquid Hydrogen as a Fuel for the Future. Science 1971, 174, 367.
- Winsche, W.E.; Hoffman, K.C.; Salzano, F.J. Hydrogen: Its Future Role in the Nation’s Energy Economy. Science 1973, 180, 1325–1332.
- Momirlan, M.; Veziroglu, T.N. The properties of hydrogen as fuel tomorrow in sustainable energy system for a cleaner planet. J. Hydrog. Energy 2005, 30, 795–802.
- Bockris, J.O.M. The hydrogen economy: Its history. J. Hydrog. Energy 2013, 38, 2579–2588.
- Abbas, S.A.; Ma, A.; Seo, D.; Lim, Y.J.; Jung, G.D.; Nam, K.M. Application of Spiky Nickel Nanoparticles to Hydrogen Evolution Reaction. Kor. Chem. Soc. 2020, 41, 1080–1085.
- Armaroli, N.; Balzani, V. The Hydrogen Issue. ChemSusChem 2011, 4, 21–36.
- Kim, J.H.; Kim, C.; Jeon, Y.; Kim, S. Hydrogen Production from Makgeolli Wastewater Using a Single‐Chamber Microbial Electrolysis Cell. Korean Chem. Soc. 2020 41, 150–155.
- Dalebrook, A.F.; Gan, W.; Grasemann, M.; Moret, S.; Laurenczy, G. Hydrogen storage: Beyond conventional methods. Commun. 2013, 49, 8735–8751.
- Felderhoff, M.; Weidenthaler, C.; von Helmolt, R.; Eberle, U. Hydrogen storage: The remaining scientific and technological challenges. Chem. Chem. Phys. 2007, 9, 2643–2653.
- Grochala, W.; Edwards, P.P. Thermal Decomposition of the Non-Interstitial Hydrides for the Storage and Production of Hydrogen. Rev. 2004, 104, 1283–1316.
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. J. Hydrog. Energy 2007, 32, 1121–1140.
- Chahine, R.; Bose, T.K. Low-pressure adsorption storage of hydrogen. J. Hydrog. Energy 1994, 19, 161–164.
- Amankwah, K.A.G.; Noh, J.S.; Schwarz, J.A. Hydrogen storage on superactivated carbon at refrigeration temperatures. J. Hydrog. Energy 1989, 14, 437–447.
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen Storage in Microporous Metal-Organic Frameworks. Science 2003, 300, 1127–1129.
- DOE Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles. Available online: https://energy.gov/eere/fuelcells/doe-technicaltargets-onboard-hydrogen-storage-light-duty-vehicles (accessed 30 March 2017).
- Modisha, P.M.; Ouma, C.N.M.; Garidzirai, R.; Wasserscheid, P.; Bessarabov, D. The Prospect of Hydrogen Storage Using Liquid Organic Hydrogen Carriers. Energy Fuels 2019, 33, 2778–2796.
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid organic hydrogen carriers for transportation and storing of renewable energy – Review and discussion. Power Sources 2018, 396, 803–823.
- Crabtree, R.H. Nitrogen-Containing Liquid Organic Hydrogen Carriers: Progress and Prospects. ACS Sustain. Chem. Eng. 2017, 5, 4491–4498.
- Wild, V.J., Friedrich, T.; Cooper, A.; Toseland, B.; Muraro, G.; Tegrotenhuis, W.; Wang, Y.; Humble, P.; Karim, A. Liquid Organic Hydrogen Carriers (LOHC): An auspicious alternative to conventional hydrogen storage technologies. In Proceedings of the 18th World Hydrogen Energy Conference, Essen, Germany, 16–20 May 2010; Volume 78, pp. 189–197.
- Cooper, A.C.; Campbell, K.M.; Pez, G.P. An integrated hydrogen storage and delivery approach using organic liquid-phase carriers. In Proceedings of the 16th World Hydrogen Energy Conference, Lyon, France, 13–16 June 2006; pp. 1–12.
- Taube, M.; Rippin, D.W.T.; Cresswell, D.L.; Knecht, W. A system of hydrogen-powered vehicles with liquid organic hydrides. J. Hydrog. Energy 1983, 8, 213–225.
- Klvana, D.; Chaouki, J.; Kusohorsky, D.; Chavarie, C.; Pajonk, G.M. Catalytic storage of hydrogen: Hydrogenation of toluene over a nickel/silica aerogel catalyst in integral flow conditions. Catal. 1988, 42, 121–130.
- Itoh, N.; Xu, W.C.; Hara, S.; Sakaki, K. Electrochemical coupling of benzene hydrogenation and water electrolysis. Today 2000, 56, 307–314.
- Kariya, N.; Fukuoka, A.; Ichikawa, M. Efficient evolution of hydrogen from liquid cycloalkanes over Pt-containing catalysts supported on active carbons under “wet–dry multiphase conditions”. Catal. A 2002, 233, 91–102.
- Pez, G.P.; Scott, A.R.; Cooper, A.C.; Cheng, H. Hydrogen storage reversible hydrogenated of pi-conjugated substrates. U.S. Patent 7,101,530 B2, 4 November
- Sotoodeh, F.; Smith, K.J. Kinetics of Hydrogen Uptake and Release from Heteroaromatic Compounds for Hydrogen Storage. Eng. Chem. Res. 2010, 49, 1018–1026.
- Sobota, M.; Nikiforidis, I.; Amende, M.; Zanón, B.S.; Staudt, T.; Höfert, O.; Lykhach, Y.; Papp, C.; Hieringer, W.; Laurin, M.; et al. Dehydrogenation of Dodecahydro-N-ethylcarbazole on Pd/Al2O3 Model Catalysts. Eur. J. 2011, 17, 11542–11552.
- Sotoodeh, F.; Huber, B.J.M.; Smith, K.J. Dehydrogenation kinetics and catalysis of organic heteroaromatics for hydrogen storage. J. Hydrog. Energy 2012, 37, 2715–2722.
- Campbell, P.G.; Zakharov, L.N.; Grant, D.J.; Dixon, D.A.; Liu, S.-Y. Hydrogen Storage by Boron−Nitrogen Heterocycles: A Simple Route for Spent Fuel Regeneration. Am. Chem. Soc. 2010, 132, 3289–3291.
- Luo, W.; Zakharov, L.N.; Liu, S.-Y. 1,2-BN Cyclohexane: Synthesis, Structure, Dynamics, and Reactivity. Am. Chem. Soc. 2011, 133, 13006–13009.
- Liu, S.-Y. Hydrogen Storage by Novel CBN Heterocycle Materials. Final Rep. 2015, doi:10.2172/1221989.
- Crabtree, R.H. Hydrogen storage in liquid organic heterocycles. Energy Environ. Sci. 2008, 1, 134–138.
- Müller, K.; Völkl, J.; Arlt, W. Thermodynamic Evaluation of Potential Organic Hydrogen Carriers. Energy Technol. 2013, 1, 20–24.
- Okada, Y., Mikuriya, T.; Yasui, T.M. Large scale hydrogen energy storage transportation technology. “SPERA” system. Enjiniyaringu 2015, 60, 187–193.
- Teichmann, D.; Stark, K.; Müller, K.; Zöttl, G.; Wasserscheid, P.; Arlt, W. Energy storage in residential and commercial buildings via Liquid Organic Hydrogen Carriers (LOHC). Energy Environ. Sci. 2012, 5, 9044–9054.
- Haupt, A.; Müller, K. Integration of a LOHC storage into a heat-controlled CHP system. Energy 2017, 118, 1123–1130.
- Eypasch, M.; Schimpe, M.; Kanwar, A.; Hartmann, T.; Herzog, S.; Frank, T.; Hamacher, T. Model-based techno-economic evaluation of an electricity storage system based on Liquid Organic Hydrogen Carriers. Energy 2017, 185, 320–330.
- Niermann, M.; Drünert, S.; Kaltschmitt, M.; Bonhoff, K. Liquid organic hydrogen carriers (LOHCs) – techno-economic analysis of LOHCs in a defined process chain. Energy Environ. Sci. 2019, 12, 290–307.
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-free Hydrogen Economy. Chem. Res. 2017, 50, 74–85.
- Gianotti, E.; Taillades-Jacquin, M.; Rozière, J.; Jones, D.J. High-Purity Hydrogen Generation via Dehydrogenation of Organic Carriers: A Review on the Catalytic Process. ACS Catal. 2018, 8, 4660–4680.
- Biniwale, R.B.; Rayalu, S.; Devotta, S.; Ichikawa, M. Chemical hydrides: A solution to high capacity hydrogen storage and supply. J. Hydrog. Energy 2008, 33, 360–365.
- Zhu, Q.-L.; Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 2015, 8, 478–512.
- Clot, E.; Eisenstein, O.; Crabtree, R.H. Computational structure–activity relationships in H2 storage: How placement of N atoms affects release temperatures in organic liquid storage materials. Commun. 2007, 2231-2233.
- Wang, Z.; Belli, J.; Jensen, C.M. Homogeneous dehydrogenation of liquid organic hydrogen carriers catalyzed by an iridium PCP complex. Faraday Discuss. 2011, 151, 297–305.
- Yamaguchi, R.; Ikeda, C.; Takahashi, Y.; Fujita, K.-I. Homogeneous Catalytic System for Reversible Dehydrogenation−Hydrogenation Reactions of Nitrogen Heterocycles with Reversible Interconversion of Catalytic Species. Am. Chem. Soc. 2009, 131, 8410–8412.
- Shimbayashi, T.; Fujita, K.-i. Metal-catalyzed hydrogenation and dehydrogenation reactions for efficient hydrogen storage. Tetrahedron 2020, 76, 130946.
- Boufaden, N.; Akkari, R.; Pawelec, B.; Fierro, J.L.G.; Zina, M.S.; Ghorbel, A. Dehydrogenation of methylcyclohexane to toluene over partially reduced silica-supported Pt-Mo catalysts. Mol. Catal. A Chem. 2016, 420, 96–106.
- Pande, J.V.; Shukla, A.; Biniwale, R.B. Catalytic dehydrogenation of cyclohexane over Ag-M/ACC catalysts for hydrogen supply. J. Hydrog. Energy 2012, 37, 6756–6763.
- Modisha, P.; Gqogqa, P.; Garidzirai, R.; Ouma, C.N.M.; Bessarabov, D. Evaluation of catalyst activity for release of hydrogen from liquid organic hydrogen carriers. J. Hydrog. Energy 2019, 44, 21926–21935.
- Papp, C.; Wasserscheid, P.; Libuda, J.; Steinrück, H.-P. Liquid Organic Hydrogen Carriers: Surface Science Studies of Carbazole Derivatives. Rec. 2014, 14, 879–896.
- Cooper, A.C. Hydrogen storage and delivery by reversible hydrogenation of liquid-phase hydrogen carriers. Am. Chem. Soc. 2005, 50, 271.
- Daubert, T.E.; Danner, R.P. Physical and Thermodynamic Properties of Pure Chemicals; Taylor and Francis: London, UK, 1997.
- Amende, M.; Gleichweit, C.; Schernich, S.; Höfert, O.; Lorenz, M.P.A.; Zhao, W.; Koch, M.; Obesser, K.; Papp, C.; Wasserscheid, P.; et al. Size and Structure Effects Controlling the Stability of the Liquid Organic Hydrogen Carrier Dodecahydro-N-ethylcarbazole during Dehydrogenation over Pt Model Catalysts. Phys. Chem. Lett. 2014, 5, 1498–1504.
- Fraunhofer Environmental Evaluation Methods. Toxic Potential Indicator (TPI). 2018. Available online: https://www.izm.fraunhofer.de/en/abteilungen/environmental_reliabilityengineering/key_research_areas/environmental_assessmentandecodesign/toxic-potential-indicator–tpi-.html (accessed on 19 June 2018).
- Brückner, N.; Obesser, K.; Bösmann, A.; Teichmann, D.; Arlt, W.; Dungs, J.; Wasserscheid, P. Evaluation of Industrially Applied Heat-Transfer Fluids as Liquid Organic Hydrogen Carrier Systems. ChemSusChem 2014, 7, 229–235.
- Markiewicz, M.; Zhang, Y.-Q.; Empl, M.T.; Lykaki, M.; Thöming, J.; Steinberg, P.; Stolte, S. Hazard assessment of quinaldine-, alkylcarbazole-, benzene- and toluene-based liquid organic hydrogen carrier (LOHCs) systems. Energy Environ. Sci. 2019, 12, 366–383.
- Müller, K.; Stark, K.; Emel’yanenko, V.N.; Varfolomeev, M.A.; Zaitsau, D.H.; Shoifet, E.; Schick, C.; Verevkin, S.P.; Arlt, W. Liquid Organic Hydrogen Carriers: Thermophysical and Thermochemical Studies of Benzyl- and Dibenzyl-toluene Derivatives. Eng. Chem. Res. 2015, 54, 7967–7976.