Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Analytical
Imidacloprid (IMI) has been applied in agricultural production to prevent pests. It is vital to detect IMI residues with high sensitivity for food safety. In general, nanomaterials have driven the development of highly sensitive sensing platforms owing to their unique physical and chemical properties. Nanomaterials play important roles in the construction of high-performance sensors, mainly through sample pretreatment and purification, recognition molecules immobilization, signal amplification, and providing catalytic active sites.
- imidacloprid
- nanomaterials
- chromatographic methods
- electrochemical sensors
- optical sensors
1. Introduction
In recent years, the market share of neonicotinoid pesticides has steadily increased in the global insecticide market because of their relative low toxicity to mammals but high activity against insects [1]. As a first-generation neonicotinoid pesticide, imidacloprid (IMI, 1-((6-Chloro-3-pyridiny)methyl)-N-nitro-imidazolidinimine) was first introduced in the 1990s. IMI is the largest-selling pesticide among neonicotinoid pesticides, and it is widely used for seed treatment in agriculture to protect crops from pests. It acts on the nicotinic acetylcholine receptor (nAChRs) by disturbing the central nervous systems of insects [2]. However, the long lifetime and high dose uses of IMI lead to large residues in environmental water, soil, and food, which could enter the human body through the food chain and pose a potential risk to human health [3,4,5]. This has led to some related laws being established to limit the maximum amount of IMI residue.
Up until now, various analytical methods have been developed for pesticide detection, including chromatographic techniques, electrochemical methods, and optical methods. Each of them has its own advantages and disadvantages (discussed in detail in Part 3). For example, efficient sample pretreatment methods are required before chromatographic analysis [8]. The weaker electrochemical response of IMI on traditional bare electrodes results in low sensitivity. Introducing nanomaterials into these analytical techniques can overcome these shortcomings. With the advancement of nanotechnology and synthetic methodologies, various nanostructure materials (NMs), including carbon-base materials (graphene, carbon nanotubes, porous carbon, etc.), metal nanoparticles, metal oxides and sulfides, upconverting nanoparticles, metal-organic frameworks (MOFs), conducting polymers, and their composites, have been designed and prepared [9,10,11,12]. These nanostructure materials possess excellent physicochemical and plasmonic properties owing to their small particle size and high surface area. These properties make them an essential part of the field of analytical techniques [13,14,15,16]. Nanomaterials play roles in sensor construction by enhancing catalytic activity, immobilizing biological entities (enzyme, antibody, aptamer, etc.), signal amplification, purification, exaction, and separation [17,18,19,20,21].
2. The Metabolites of IMI
When pesticides are applied to crops, they are often converted into metabolites. In the case of IMI, the degradation of IMI produces several metabolites, including imidacloprid-olefin (IMI-olefin), urea-imidacloprid (IMI-urea), 6-chloronicotinic acid (6-CNA, or 6-chl), 5-hydroxy-imidacloprid (5-OH-IMI), desnitro-imidacloprid (DN-IMI), and nitro-methylene analogue (CH-IMI) [25,26,27,28,29,30], etc. Their chemical structures are shown in Figure 1. The metabolites produced by IMI are related to the environment, and they exhibit higher toxicity than IMI. For example, in casing soil during mushroom cultivation, the main metabolites were IMI-urea, IMI-olefin, and 6-CNA [31]. In honey, the concentrations of IMI-olefin (5.6 ng g−1) and 5-OH-IMI (21.1 ng g−1) were higher than those of IMI (0.8 ng g−1) [32]. To make matters worse, the metabolites are more toxic than IMI itself. For example, IMI-olefin is twice as toxic as IMI [33]. Hence, the risk of IMI metabolites should be considered comprehensively when evaluating the harm of IMI.
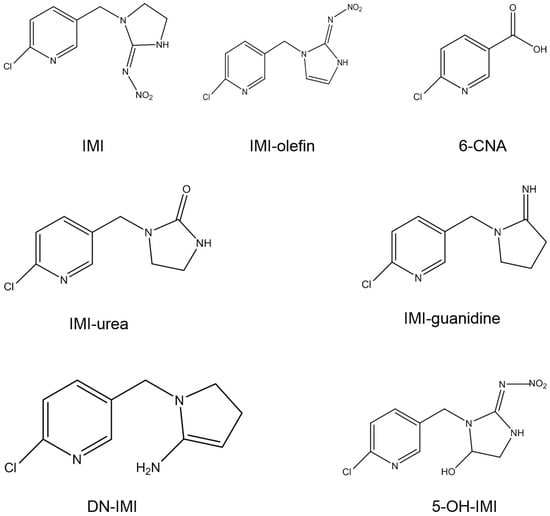
Figure 1. Chemical structures of IMI and its metabolites.
3. Detection Methods
3.1. Sample Pretreatment and Chromatographic Analysis
The traditional chromatographic methods, including gas chromatography (GC), high-performance liquid chromatography (HPLC), mass spectrometry (MS), gas chromatography-tandem mass spectrometry (GC-MS), high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS), capillary electrophoresis-mass spectrometry (CE-MS), and ultraperformance liquid chromatography/tandem mass spectrometry (UPLC-MS/MS), can offer high accuracy and have been recognized as the gold standard for the detection of many organic analytes [34,35,36,37]. However, owing to the wide use of insecticides, they may exist in a variety of complex matrices, such as soil, food, Chinese medicine, etc., and the interferences in these matrices often affect the accuracy and sensitivity of detection [38]. Therefore, the determination of pesticide residues generally includes two steps: sample pretreatment and quantitative detection. Up to now, researchers have proposed versatile sample pretreatment methods to extract targets from different samples, such as solvent extraction, solid phase extraction (SPE), the QuEChERS method (the Quick, Easy, Cheap, Effective, Rugged, and Safe method), etc. [8,39,40,41,42]. Nanomaterials could be used for the extraction and enrichment of targets in complex matrices due to their small size, large specific surface area, and adjustable surface groups.
3.2. Electrochemical Sensors
Recently, various electrochemical techniques have attracted much attention from researchers due to their numerous advantages, including high sensitivity, low cost, and easy preparation [60]. Hence, electrochemical methods have been used for constructing sensors for IMI. The electrochemical detection performance of IMI is mainly dependent on various electrode-modified materials, such as carbon composite, reduced graphene oxide, β-cyclodextrin, bismuth film, silica film, molecularly imprinted polymers, biological materials, and so on. There are mainly three electrochemical strategies for IMI detection: direct detection, indirect detection (electrochemical sensors based on MIPs and biometric recognition elements), and electrochemical ratio sensors.
3.2.1. Direct Electrochemical Detection
IMI is an electroactive molecule in which the nitro group can undergo two-electron or four-electron reduction on an electrode surface. The direct electrochemical reduction of IMI proceeded on various modified electrodes to improve the sensitivity. β-cyclodextrin (β-CD) has a hydrophobic internal cavity and a hydrophilic external surface, which make it useful for capturing various molecules. Therefore, β-CD was extensively applied for sensor construction. For instance, Pereira [61] developed an electrochemical sensor for IMI based on β-CD film coated on a glass carbon electrode (GCE). The electrochemical performance of IMI on β-CD/GCE was better than that on bare GCE due to the encapsulating effect of β-CD for IMI. A variety of carbon materials, including carbon nanotubes, carbon paste, graphene oxide (GO) or reduced graphene oxide (rGO), etc., have excellent electrochemical properties and are widely used in electrochemical sensors.
3.2.2. Electrochemical Sensors Based on MIPs
Molecularly imprinted polymers (MIPs) are tailor-made synthetic materials with high-affinity binding sites for a specific target molecule [73]. MIPs are prepared by mixing a target molecule as a template with a cross-linking agent and an initiator. After polymerization, the template is removed, leaving the hole exactly the same as the target molecule. The forming hole can rebind perfectly with the target molecule, allowing it to be specifically recognized and detected target molecule. Therefore, sensors based on MIPs have been designed and applied in various fields, such as environmental analysis [74], pharmaceutical analysis [75], nucleic acid assay [76], and food safety [77].
3.2.3. Electrochemical Sensor Based on Biometric Molecules
Except for MIP, some biometric recognition elements such as antibodies, enzymes, and aptamers have been used to improve the selectivity of sensors. Timur et al. [82] found an aptamer of IMI by the Systematic Evolution of Ligands by Exponential Enrichment (SELEX) process, and an aptasensor was constructed for IMI detection in a range of 0.1–50 ng mL−1 with a LOD of 0.19 ng mL−1. Pérez-Fernández et al. [83] reported a competitive immunosensor for IMI detection based on AuNPs-modified screen-printed carbon electrodes (AuNPs-SPCE). In this work, a monoclonal antibody to IMD (mAb-IMD) was immobilized on AuNPs-SPCE, and a competitive assay was performed between free IMI and IMI labeled with horseradish peroxidase (HRP). The electrochemical reduction signal of oxidized 3,3’,5,5’-tetramethylbenzidine (TMB) was associated with IMI concentration, avoiding the use of secondary antibodies. This sensor exhibited excellent performance for IMI determination, with a satisfactory low LOD, high selectivity, and stability. Furthermore, this sensor was successfully applied in IMI analysis on a real sample, and the reliability was also validated by HPLC-MS/MS.
3.2.4. Ratiometric Electrochemical Sensor
The classical electrochemical sensor contains only a single electrochemical signal of the target molecule, and its reproducibility is easily influenced by electrode properties or the complex detection system. To overcome this limitation, ratiometric electrochemical sensors involving the simultaneous measurement of two electrochemical signals at different potentials have been developed. By introducing a built-in correction for the analyte’s signal, the ratio electrochemical sensor greatly improves the reproducibility and reliability of electrochemical detection. So far, ratio electrochemical sensors have been used to detect metal ions, nucleic acids, proteins, biological small molecules, etc. [86,87]. The researchers also applied the ratio sensing strategy for IMI detection. The Kan group [88] constructed a ratio electrochemical sensor by electropolymerization of thionine and β-CD composite on GCE for IMI determination, in which thionine as an internal reference element provides a built-in correction. The current ratio of IMI and thionine was employed as the signal for IMI detection, and it exhibited a good linear relationship in the concentration range of 0.04–10 μM.
To sum up, researchers have developed a variety of electrochemical sensing strategies based on nanomaterials for IMI detection. On the one hand, designing and synthesizing nanomaterials with controlled morphology or preparing hybrid materials to further improve the electrocatalytic reduction of IMI is highly necessary. On the other hand, combining the advantages of easy miniaturization of electrochemical sensors with specific recognition elements, the establishment of hand-held electrochemical sensing devices with high selectivity and sensitivity has great prospects.
3.3. Optical Sensors
Over the past decades, researchers have devoted intensive efforts to developing various optical sensors due to their advantages, including simplicity, ultra-sensitivity, and high selectivity. The principle of an optical sensor is based on the shift of the characteristic signal caused by the interaction of the analyst with the substrate or with other optical molecules. With the development of nanotechnology, many optical detection platforms have been born. According to the different detection signals, multiple optical sensors, including fluorescence, colorimetry, surface plasmon resonance (SPR), and surface enhanced Raman spectroscopy (SERS), have been applied to detect IMI.
3.3.1. Fluorescent Method
Recently, with the significant development of optical nanomaterials, fluorescence sensing has dramatically benefited from various luminescent nanoparticles. For instance, Tian et al. [90] developed two kinds of lateral flow immunoassay (LFIA) for IMI determination based on time-resolved fluorescent nanobeads and colloidal gold, respectively. The proposed LFIAs achieved high accuracy and a low LOD for IMI analysis. Guo et al. [12] established a competitive fluorescence resonance energy transfer (FRET) immunoassay for IMI detection. FRET occurred through the specific immunoreaction between antigen/GO and mAb/up-converting nanoparticles, (UCNPs). The fluorescence intensity of UCNPs was weakened by GO, and the florescent recovery of UCNPs is associated with IMI concentration through the competitive mechanism. This sensor showed a wide range of 0.08–50 ng/mL for IMI in the presence of other interferences.
3.3.2. Colorimetric and Surface Plasmon Resonance (SPR) Sensors
A colorimetric sensor has the merits of visualization, simplicity, low cost, and being a powerful tool for high-throughput analysis. In recent years, various noble metal nanoparticles, such as AuNPs and AgNPs, have been applied for the construction of colorimetric sensors owing to their strong localized surface plasmon resonance (LSPR) effect. Further, plasmonic colorimetric sensors based on metal nanoparticles have been applied to detect various analytes, including metal ions, pesticides, proteins, DNA, pathogens, and so on [92,93,94,95,96].
3.3.3. Surface-Enhanced Raman Spectroscopy
The Surface Enhance Raman Scattering (SERS) technique has received much attention in the field of analysis due to its non-invasive and unique fingerprint characteristics. In particular, the Raman signal can be enhanced with the use of nanomaterials with plasmonic properties, greatly improving the sensitivity of detection. For example, the O’Riordan group [100] proposed a SERS sensor for two neonicotinoids, including clothianidin and IMI, using Ag nanoparticles coated Polyvinylidene fluoride (PVDF) substrates. The developed SERS can sense 1 ng/mL IMI with a LOD of 4 nM.
This entry is adapted from the peer-reviewed paper 10.3390/chemosensors11050300
This entry is offline, you can click here to edit this entry!
