The intrinsic characteristics and the complex composition of honey, in which different substances with antimicrobial properties are included, make it an antimicrobial agent with multiple and different target sites in the fight against bacteria. This characteristic could be behind the difficulty of bacteria to acquire honey-resistance. and suggests that it could become an effective alternative in the treatment of antibiotic-resistant bacteria.
- natural antibiotic
- antibacterial action mechanisms
- antibiotic-resistant bacteria
1. Introduction
In 1945, Alexander Fleming, Howard Florey, and Ernst Chain were awarded the Nobel prize for the discovery of the first broad-spectrum antibiotic in history: penicillin. Soon after this finding, they realized and warned of the ease with which bacteria could develop tolerance to that new remedy if it was misused. Today, antimicrobial resistance has become a challenging situation, not only for human health, but also for human-connected animals, farms, food, water, and natural ecosystems worldwide. Antibiotic resistance is a natural phenomenon that occurs when bacteria are exposed to antibiotics. Under the drug selective pressure, susceptible bacteria are killed or inhibited, while those bacteria that are naturally resistant, or that have acquired antibiotic-resistant features, have more opportunities to survive and multiply. The overuse and improper use of antibiotics amplify and accelerate this circumstance.Currently, there are bacteria which are able to resist almost all, or even all, the approved antimicrobial agents for their treatment. Consequently, some common infections have become very difficult, or even impossible, to treat. The cost of treating antibiotic-resistant infections is much higher than that of treating susceptible ones; the rise in the costs of the antibiotic therapy, derived from the necessity of using more expensive antibiotics, is coupled with a lengthier stay in hospital.Further to the economic impact, an increase in mortality is another consequence of antibiotic resistance. Recent estimations based on data from EARS-Net (European Antimicrobial Resistance Surveillance Network) show that each year, approximately 33,000 people in Europe die as a direct consequence of infections caused by bacteria resistant to antibiotics. Therefore, the research and development of a new generation of antimicrobials to alleviate the expansion of antibiotic resistance has become a priority. However, testing a new antimicrobial before commercialization requires long time periods. Moreover, the current strategies to limit the appearance of resistance and to safeguard the efficacy of new antimicrobials entail a significant obstacle in drug development. Limiting their use, as much as possible, to those cases that do not respond successfully to existing products leads to a lack of profitability for pharmaceutical companies. In this way, the development of new antimicrobials is no longer an interesting activity. Faced with this situation, the search for new alternatives has led to the scientific study of substances, formulas or active ingredients used before the antibiotic era. Honey has been traditionally used not only as a food but also with therapeutic purposes. One of the most common curative applications is the topical treatment of wounds, especially for its antimicrobial properties. However, with the advent of antibiotics, the use of honey gradually declined. In recent decades, more and more scientific studies have been focused on revealing which compounds present in honey are related to its antibacterial activity, and the action mechanisms through which honey kills bacteria. The effectiveness of manuka honey as an antimicrobial agent has been extensively studied; for this reason, it has long been regarded as one of the most efficacious honey varieties known. However, several studies using other honey varieties also demonstrated promising antibacterial properties, and prove similar, or even greater, efficacy than manuka honey. Despite the multiple studies on the antibacterial properties of honey, its use as an antibacterial agent continues to be underestimated.
2. How Honey Acts against Bacteria: Antibacterial Action Mechanisms
Despite the numerous studies on the antibacterial properties of honey, the lack of comprehensive evidence explaining the mechanisms through which honey interferes with bacteria, limits, partly, its application as an antibacterial agent. Honey is a very complex substance, containing hundreds of compounds that cause specific and distinct effects on microorganisms (Figure 1). The mode of action of honey against bacteria differs among Gram-positive and Gram-negative microorganisms, some studies hypothesized that at least some cellular targets might be broadly specific for each class of bacteria. However, given the complexity of the matrix, there are probably several mechanisms that have not been described yet.
In terms of understanding the mechanisms of action of honey upon bacteria, most of the research has been undertaken using manuka honey. Nevertheless, more and more studies are being carried out with other, different varieties (Figure 1).
2.1. Structural and Morphological Changes
One of the earlier attempts to reveal honey’s action mechanisms were centred on observable characteristics by using scanning and transmission electron microscopy. In methicillin-sensitive Staphylococcus aureus and methicillin-resistant S. aureus (MRSA), it was verified that manuka honey did not induce a significant cellular lysis, few surface changes were found and the majority of the cells retained a smooth surface after four hours of treatment. On the contrary, Pseudomonas aeruginosa cells exposed to manuka honey exhibited widespread structural damage and large membrane bubbles, which led to cell lysis and bacterial death. This result was verified by genomic analysis, showing that manuka honey treatment causes a reduction in the expression of OprF, an integral membrane protein that is essential for the structural stability of the cell envelope in Gram-negative bacteria. After these studies, it was found that manuka and kanuna honey exerted very different changes on the cellular morphology of Bacillus subtilis, Escherichia coli, S. aureus, and P. aeruginosa. While for the first three bacteria a significant cell shortening was observed, in the case of P. aeruginosa, cells appeared longer than usual, which is possibly a reflection of the cell envelope damage that likely happened. More recently, flow cytometry also confirmed the previous results. The treatment time and the honey concentration were identified as key factors in the induction of membrane injury in S. aureus and E. coli cells. The differences observed among Gram-positive and Gram-negative bacteria were also highlighted in this study. Membrane permeabilization after manuka honey exposition was also verified by flow cytometry assays in P. aeruginosa.
These mechanisms are not exclusively attributable to manuka honey, and several honey varieties have been demonstrated to produce morphological and structural alterations on bacteria as one of their first effects (Figure 1). Buckwheat honey and wildflower honey were demonstrated to induce a rapid disruption of the cell wall of E. coli, which was honey concentration- and treatment time-dependent. Structural and morphological changes, such as altered shape, modified surface layers, cellular debris, increased vacuoles, and/or with irregular shrunken cytoplasm, as well as the presence of electron dense material, were also described in E. coli cells after treatment with citrus, clover and marjoram honey. Agastache honey caused extensive cell lysis and membrane disruption in MRSA and E. coli. Avocado, chestnut and polyfloral honey samples induced membrane injury upon S. aureus and E. coli with differences among bacterial types. While the effect of the different honey samples on S. aureus membrane integrity was limited, in the case of E. coli cells, the results obtained confirm a more meaningful damage. Under similar treatment conditions, these three honey samples caused more relevant changes in membrane integrity than manuka honey. On the other hand, no morphological changes in P. aeruginosa cells were observed after Corsican honey exposure, despite the fact that this bacterium was sensitive to the different varieties tested.
In addition, related to changes in bacterial morphology, it was verified that manuka honey suppresses P. aeruginosa flagellum gene expression, by impacting on regulatory and structural genes, which entails a reduction in protein expression, and means a significant reduction in flagellated cells. This finding has consequences beyond morphological alterations in bacterial cells, since flagellum is critical for pathogens to establish and produce invasive infection, so honey can also reduce the pathogenic potential of infecting bacteria.
2.2. Alterations of Bacterial Membrane Potential
Membrane potential plays an essential role in several bacterial physiological processes. The loss of the equilibrium of ion concentration inside and outside of bacteria may affect to their viability.
Three recent studies described, for the first time, that different honey varieties (manuka, avocado, chestnut and a polyfloral honey) induce significant membrane depolarization in P. aeruginosa, S. aureus, and E. coli. This finding has great significance, since a collapse in the membrane potential means that bacteria are not able to generate the energy required to carry out their normal physiological processes, such as the correct spatial organization of cell division proteins and regular cell division function, as well as to drive the mechanisms necessary for its survival, such as some multidrug efflux pumps (Figure 1).
2.3. Bacterial Cell Cycle and Cell Growth Changes
The first studies using scanning and transmission electron microscopy revealed that the main effect of manuka honey upon methicillin-sensitive S. aureus and MRSA involved the interruption of the cell cycle. Bacterial cells were unable to separate, which resulted in an accumulation of arrested cells with a fully formed septum. It was suggested that septa were formed prematurely in the cell cycle, and cell division was then interrupted because mandatory cellular events had not been completed yet. Alternatively, cell division might have been prevented due to the effects of manuka honey on autolysin activity, which normally controles the septum rupture to produce the two daughter cells.
In addition, it was corroborated that manuka honey induced a dose-dependent extension of the lag phase of cell growth of B. subtilis, E. coli, and S. aureus when they were treated with manuka honey. This growth behavior was also observed when these bacterial cultures were exposed to MGO, and was not detected after the treatment of bacteria with other honey varieties (clover or kanuka honey); so, the extended duration of lag phase was supposed to be due to MGO. In contrast, P. aeruginosa showed a markedly different pattern of growth inhibition to the other three bacteria; very little or no lag-phase extension was observed after honey treatment, except for the treatment with clover honey.
Finally, honey also affects bacterial DNA. This effect, observed on B. subtillis and E. coli, was related to the interactions of H2O2 with polyphenols as active intermediates that are necessary to confer oxidative action of H2O2 through hydroxyl radical production. This mechanism was supported by transmission electron microscopy observations, which revealed “ghost” E. coli cells lacking DNA, after citrus honey treatment . Moreover, exponentially growing P. aeruginosa cells presented condensed chromosomes after treatment with manuka honey, which suggest that this variety of honey inhibits DNA replication in these cells by non-oxidative stress mechanisms.
2.4. Disruption of Bacterial Metabolism
Despite the fact that very few studies have been carried out to evaluate the effect of honey on the metabolic activity of bacteria, the concordance of the results obtained indicates that honey plays a key role in the metabolic disruption of both Gram-positive and Gram-negative bacteria. Genomics and proteomics demonstrated that manuka honey is able to reduce the expression of genes and proteins involved in the energy metabolism of MRSA. These findings were corroborated by other flow cytometry assays to evaluate the repercussion upon metabolic bacterial physiology of reference strains of S. aureus and E. coli induced by manuka honey, and also by other three honey varieties (avocado, chestnut and polyfloral). However, differences in bacterial response, depending on the honey type and the concentration tested, were observed, probably due chemical composition variances among samples. Metabolic disruption induced by manuka honey seemed to be an irreversible effect, whereas in the case of the other honey varieties, bacteria appear to reprogram their metabolism in response to the environmental stress, being able to progressively recover metabolic activity, at least partially, at the end of honey exposure.
Moreover, other underlying mechanisms could also affect bacterial metabolism. Studies on E. coli, as well as on P. aeruginosa and S. aureus, confirmed the effect of manuka honey as an iron-chelator, generating a limiting environment of this element, which is essential for bacterial metabolism and survival. Furthermore, membrane potential is also a fundamental process in energy generation for bacteria. It has been described that honey induces membrane depolarization, which may impede the ability of bacteria to generate the energy required for several processes.
2.5. Effect on Efflux Pumps Activity
Multidrug efflux pumps are cell membrane glycoproteins that can eject different classes of compounds across bacterial membranes; for this reason, they are one of the most important mechanisms of drug resistance, since antibiotics are not capable of reaching the concentration at which they are active within the bacteria.
Some natural products, including plant extracts, essential oils or isolated compounds, are capable of inhibiting or modifying the efflux pumps’ activity. In this context, manuka honey has been proven to induce alterations in the expression of genes belonging to the evgAS regulon, related to bacterial adaptive responses to acid, osmotic and drug resistance. The results obtained in this first study were partly corroborated by later research; another study using different honey types (clover, citrus and marjoram) demonstrated that depending on the honey variety, the gene expression profile was different. EvgA expression was upregulated after the treatment with clover honey, but it was downregulated after citrus or marjoram honey-exposure. The dissimilarities in the expression patterns may reflect compositional differences among honey varieties and/or differences in their action mechanisms, since the major antimicrobial activity of manuka honey is not related to H2O2, while the other honey varieties were shown to be mainly peroxide-dependent.
A more recent study, using flow cytometry, proved that manuka honey was able to disrup efflux pump activity of an AG100 progeny strain of E. coli, which over-expresses several efflux pumps when exposed to high concentrations of tetracycline. Manuka honey blocked efflux pump activity, following a dose-dependent tendency. This finding seems to differ from those previously described. These discrepancies may be justified by a lack of correlation between gene expression and bacterial physiological response or by other mechanisms not directly related to the genes involved in bacterial defence and adaptative reponse. It was suggested that this effect could be associated with the metabolic disruption, since antibiotic efflux is an energy-dependent mechanism. More recently, Bouza et al. hypothesized that manuka honey could increase the drug uptake due to the membrane potential collapse, and the increase in membrane permeabilization, which together would enhance the potency of antibiotics. These mechanisms justify why manuka honey is able to restore the tetracycline antibiotic activity against bacterial strains that would otherwise be resistant.
2.6. Bacterial Quorum Sensing Alterations
Quorum sensing is the cell to cell communication system used to coordinate and regulate the behavior of cell populations. Many bacterial physiological functions, such as luminescence, virulence, motility, sporulation or biofilm formation, are regulated by quorum sensing systems. For this reason, the interruption of bacterial quorum sensing is recognized to alleviate virulence, and is considered to be a potential new alternative to treat infections caused by pathogenic bacteria.
Only a few studies on the effect of honey on inhibition of quorum sensing have been published. Nevertheless, the results demonstrate that some genes involved in this bacterial interconnection mechanism, as well as other virulence genes, are downregulated following exposure to honey. Furthermore, honey was able to enhance the anti-quorum sensing activity of other substances, such as curcumin, when they were used in combination.
Additionally, the secretion of N-acyl-l-homoserine lactones and other quorum sensing regulating or signalling substances (pyocyanin, pyochelin and pyoverdine) is significantly reduced in response to honey exposure. It was suggested that this inhibitory activity seems to be related to the aqueous phase of the honey instead of either the total or individual phenolic content (Figure 1). In addition, pyocyanin, pyochelin and pyoverdine act as iron-chelating molecules or siderophores, which are central to bacterial proliferation in the host environment, and are inherently linked to pathogen virulence. Honey-treatment mediates a marked reduction in siderophore production, which is accompanied by the reduction in the expression of genes involved in quorum sensing. This fact demonstrates the global impact that gene downregulation exerts on the expression of virulence factors.
2.7. Biofilm Inhibition
Biofilms constitute an extraordinary microbial drug-resistance mechanism, which is composed of an assemblage of microbial cells that are irreversibly associated with a surface and enclosed in a matrix of self-produced polysaccharide material. Included in biofilms, bacteria are several times more resistant to antibiotics than planktonic cells, which makes them extremely difficult to destroy.
The effects of different varieties of honey samples on bacterial biofilms have been assessed in various studies, and the results indicate that honey is effective in disturbing the biofilm strengthening in drug-sensible and drug-resistant bacteria.
First of all, honey is able to prevent the formation of biofilms, from both Gram-negative and Gram-positive bacterial species, most likely via a non-specific mechanism such as the inhibition of bacterial growth, and the reduction in the biofilm biomass, which are consequences of the osmotic stress, coupled with the low pH and the presence of H2O2 of honey (Figure 1). This effect is dependent on concentration—when honey concentration is not enough, the biofilm formation could be enhanced.
In addition, when the biofilm is already established, honey significantly reduces its metabolic activity, probably due to the disruption of the metabolic activity of individual bacterial cells—this mechanism is also suggested to explain the inhibition of biofilm development when it is forming In this phase, honey also decreases the number of viable cells .
Finally, gene expression analysis also confirmed the effect of honey on the expression of different genes related to the formation and the development of biofilms. Honey might prevent the colonization of host tissues due to the downregulation of genes encoding binding proteins, and the inhibition of the expression of genes encoding a glucosamine polymer.
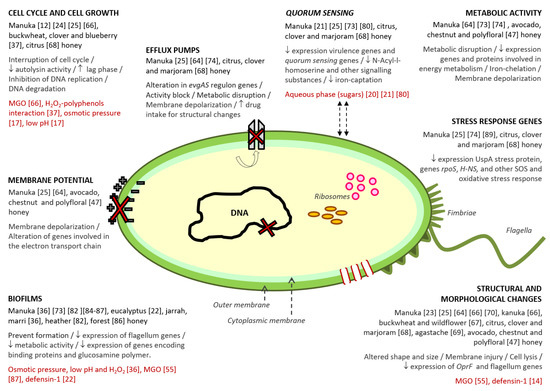
Figure 1. Antibacterial action mechanisms described for honey. Within each mechanism, the following information has been collected: (i) varieties of honey in which the mechanism was detected; (ii) the different effects related to the mechanism; (iii) the compound/s or honey characteristics associated to the mechanism, and (iv) the reference number of the studies from which the information was extracted.
2.8. Effects on Bacterial Stress Response
Several studies have demonstrated that honey exposure also affects the expresion of different genes involved in bacterial stress response. Manuka honey exposure generates the downregulation of the universal stress protein UspA in MRSA, which reduces the ability of bacteria to survive under cellular and metabolic stress conditions.
In addition, other studies have demonstrated similar effects in genes related to cell stress survaival mechanisms, namely rpoS and H-NS, after the exposure of E. coli cells to citrus, clover, and marjoram honey samples. These results were in contrast to those previously described by Blair et al., who showed marked upregulation in the same genes of E. coli following manuka honey-treatment. A more recent study demonstrated that manuka honey induced strong upregulation in a wide range of genes involved in the emergencyand the oxidative stress response of P. auregonisa cells.
Taking into account all these results, it is feasible to think that the variances in expression pattern may be justified by the differences in the antimicrobial mechanisms of the honey varieties tested, and the variable effects they can induce on certain genes.
This entry has been extracted from the review "Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria?" published in the MDPI journal Antibiotics (doi:10.3390/antibiotics9110774).
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics9110774
