After the three worldwide influenza outbreaks in the 20th century that were named in relation with the cite of origin (Spanish, 1918; Asian, 1957; Hong Kong, 1968), characterized by the infection of three different subtypes of influenza A virus (H1N1, H2N2, and H3N2, respectively) [
1], the world recently faced the ongoing pandemic at the end of 2019, since Chinese health authorities informed of an outbreak of pneumonia of unknown etiology in Wuhan City, Hubei Province, on 31 December 2019 [
2,
3,
4,
5]. World Health Organization (WHO) established this as a public health emergency of international concern on 30 January 2020 [
6]. The unknown origin was, thereafter, recognized as a possible zoonotic transmission, starting from bat and pangolin coronaviruses, spreading across other intermediate host species [
7]. On 11 February 2020, the International Committee on Taxonomy of Viruses (ICTV) named this virus “severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)” and the WHO named the related disease “Coronavirus Infectious Disease (COVID)-19” [
8]. The SARS-CoV-2 genome consists of 29,903 nucleotides, and a phylogenetic analysis suggested that the virus is closely linked (89.1% nucleotide similarity) to a group of SARS-like coronaviruses (genus Betacoronavirus, subgenus Sarbecovirus) that had been reportedly found in animal hosts such as bats in China [
9,
10,
11,
12]. Following a global infection of SARS-CoV-2, COVID-19 was declared as a pandemic by WHO on 11 March 2020 [
13]. Several clinical symptoms of COVID-19 are common to severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), such as fever, nonproductive cough, dyspnea, myalgia, fatigue, normal or decreased leukocyte counts, and radiographic evidence of pneumonia [
2,
14]. However, as described by WHO, the progression of the disease on vulnerable populations, such as pediatric patients, older people, and pregnant women, contributes to respiratory complication, multiorgan failure, the need for mechanical ventilation, and the admission to intensive care unit (ICU), where critical stage of disease could be potentially lethal [
15]. According to the WHO data on 21 December 2022, the number of confirmed cases reached more than 650 million worldwide, with more than 6.6 million of counted deaths. These numbers are continuously updated on the dashboard of WHO (website:
https://covid19.who.int/, accessed on 21 December 2022). Standard medical care for treating COVID-19 symptoms (mainly anti-inflammatory drugs) still awaits specific tools in order to counteract the most severe forms, which could lead to death. Research is moving along and it is supported by an enormous deployment of forces in terms of discovering new strategies and drugs. Importantly, the introduction of vaccines has reduced the spread of COVID-19 infection, even though the rapid increase in viral variants has reduced the effectiveness of vaccination, leading to the need for a booster dose against new viral strains. In addition, pharmacological approaches have been tested to treating COVID-19 patients, including anti-inflammatory and antiviral drugs. As a result, the damage caused by SARS-CoV-2 infection remains a long-term concern. Therapies based on the use of exogenous cells could represent an alternative and profitable strategy [
16]. It is now known that mesenchymal stromal cells (MSCs) and their extracellular vesicles (EVs) represent a gold standard in regenerative medicine for several reasons, such as multipotent differentiation potential, immunomodulation and anti-inflammatory properties, mitochondrial transfer, and promotion of endogenous repair mechanisms [
17]. MSCs can be isolated by adult tissues such as bone marrow (BM-MSCs) or adipose tissue (AT-MSCs), but also from perinatal tissues, including placenta (PL-MSCs), umbilical cord (UC-MSCs), umbilical cord blood (UCB-MSCs), and amniotic fluid (AF-MSCs). In recent years, consistent research has been carried out on EVs secreted by MSCs, which are enriched in proteins, lipids, and nucleic acids. Moreover, EVs can be used as a vehicle for drug delivery. Interestingly, it has been observed that UC-MSCs-derived EVs present a therapeutic potential for the treatment of different diseases, including COVID-19 [
18,
19,
20].
2. Characteristics of UC-MSCs in In Vitro and Preclinical Experimental Evidence Supporting Anti-Inflammatory, Immunomodulation, and Therapeutic Potential
2.1. Adult and Perinatal MSCs: General Features
Mesenchymal stromal cells (MSCs), which derive from the inner mass of the blastocyst, have a high capacity to self-renew, have fibroblastic-like shape when cultured in plastic surface, can differentiate into mesodermal derivatives such as osteoblasts, chondrocytes, and adipocytes, and show phenotype and characteristics in accordance with the minimal criteria of the International Society for Cellular Therapy (ISCT) [
111]. They have, therefore, shed a new light on treatment of patients suffering from diseases and disorders that do not yet have a definite cure, and have a long history since their discovery to therapy applications [
112]. MSCs are present in almost all post-natal/adult organs, i.e., bone marrow [
113,
114,
115], adipose tissue [
116,
117], dental pulp [
118,
119], endometrium [
120,
121], menstrual blood [
122,
123], peripheral blood [
124], salivary gland [
125,
126], skin and foreskin [
127,
128,
129,
130], synovial fluid [
131,
132], muscle [
133,
134,
135], corneal stroma [
136,
137], heart [
138,
139], and lung [
140]. Promising sources of MSCs are represented by the extraembryonic/perinatal tissues [
141], among which there are the placenta, the chorionic and amniotic membranes [
142,
143,
144], amniotic fluid [
145], umbilical cord blood [
146], and umbilical cord stroma [
147]. Moreover, since MSCs derived from perinatal, as well as adipose tissue (AT-MSCs) and bone marrow (BM-MSCs), do not express ACE2 and TMPRSS2, this demonstrates that they are not permissive to SARS-CoV-2 infection, increasing the interest in the use of MSCs as potential therapy for COVID-19 [
148]. However, the methods for obtaining adult tissues are invasive, and the yield of cells gained after isolation is scarce (e.g., 3.5 × 10
5 to 1 × 10
6 in 1 g of adipose tissue and from 500 to 5 × 10
4 from 1 g of bone marrow aspirate [
149]). Perinatal tissues provide an interesting source of MSCs as they are usually wasted after birth and, therefore, the collection procedures are without risks for the donor and ethical issues [
150]. Importantly, umbilical cord (UC) matrix is a better source of MSCs, in terms of yields, than the umbilical cord blood and adult tissues [
151,
152,
153].
2.2. UC-MSCs Properties: Multilineage Differentiation, Immune Tolerance, Angiogenesis/Wound Healing, Matrix Remodeling, and Resistance to Hypoxia
Multilineage differentiation properties—The UC is consisted of two arteries and one vein included in a connective tissue called “Wharton’s jelly” (WJ), mainly composed of sponge-like structure woven with collagen fibers, proteoglycans, and embedded MSCs, and an outer layer of amniotic epithelium [
147,
155,
156]. Scholras focused research on molecular characterization of UC-MSCs, demonstrating that these cells express CD10, CD13, CD29, CD44, CD54, CD73, CD90, CD105, Stro-1, MHC class I (classical HLA-A, -B and -C), mesenchymal markers (vimentin, α-SMA), neuroectodermal markers (Nestin, NSE, GFAP), and early endoderaml markers (GATA-4,-5,-6, HNF4, cytokeratin-8,-18,-19), and lack the major costimulatory molecules responsible for T cell activation, specifically B7-1 (CD80) and B7-2 (CD86), hematopoietic and endothelial markers CD14, CD19, CD31, CD34, CD38, CD45, CD66b, CD80, CD86, CD106, and CD133, [
147,
157,
158,
159,
160]. UC-MSCs multipotency is formally demonstrated by their in vitro differentiation capability towards cell types of mesodermal origin (chondrocytes, adipocytes, osteoblasts, odontoblast-like cells, dermal fibroblasts, smooth muscle cells, skeletal muscle cells, cardiomyocytes) and endodermal lineages (hepatocyte-like cells, pancreatic endocrine cells), as well as ectodermal and neuroectodermal (sweat gland cells, oligodendrocytes, and dopaminergic neurons) [
147,
157,
161,
162,
163,
164,
165,
166,
167,
168,
169,
170,
171,
172,
173,
174]. UC-MSCs also feature “primitive stemness” properties due to their close relation with the embryologic phase, also maintaining the length of telomeric ends even after around 60 population doublings, and having no chromosomal mutation acquisition [
157].
Immune tolerance and anti-inflammatory properties—UC-MSCs exert immunomodulation properties [
163,
175,
176], even related to the expression and release of specific factors, such as the nonclassical HLA class I antigen, HLA-G [
157,
177], HLA-E, CD276/B7-H3, leukemia inhibitory factor (LIF), indolamine 2,3-dioxygenase-1 (IDO-1), galectin-1 (Gal-1), and heat shock protein 10/Early Pregnancy Factor (HSP10/EPF), being able to modulate or inhibit lymphocyte proliferation [
175]. These factors are involved in tolerogenic processes occurring at the fetal–maternal interface [
178,
179,
180,
181,
182,
183], permitting, in turn, the semi-allogeneic embryo to escape surveillance of the maternal immune system. Specifically, HLA-G is an inhibitory molecule involved in immune tolerance and exerts its inhibitory functions interacting with inhibitory receptors Ig-like transcript (ILT) receptors, such as ILT-2, ILT-3, and ILT-4, and killer cell immunoglobulin-like receptor (KIR), two Ig domains, and long cytoplasmic tail 4, KIR2DL4, differentially expressed by NK, T, and antigen-presenting cells [
184,
185]. Its expression is enhanced by Gal-1 [
183]. HLA-G also interacts with leukocyte immunoglobulin-like receptor subfamily B member 1 (LILRB1) expressed by CD56
brightCD16
− natural killer (NK) cells that are enriched in the uterus during pregnancy, are poorly cytotoxic, and produce low amounts of IFN-γ as compared with peripheral blood CD56
dimCD16
+ NK cells [
160].
Lastly, HSP10, commonly known as a heat shock protein of 10 KDa, mainly expressed in the inner membrane of mitochondria, is also known as early pregnancy factor (EPF), discovered in the 1970s as a factor released during pregnancy in the serum of within 24 h after fertilization, preventing T cell-rosette formation and reduction of proinflammatory cytokines release, such as TNF-α (through interaction with TLR4 expressed by macrophages) (see in [
192]).
Although the underlying mechanism is still unknown, UC-MSCs secrete different prostanoids, such as PGD2, PGF2a, and PGE2, and several molecules, including IL-1R antagonist, IL-6, IL-10, M-CSF, VEGF, TGF-β1, and B7-H4, which could contribute to the differentiation of M2 macrophages [
160]. UC-MSCs have been also involved in regulation of the monocyte/macrophage system. In particular, UC-MSCs can prevent the differentiation and maturation of monocytes toward DCs [
194]. UC-MSCs also secrete other neuroprotective, angiogenic, and antiapoptotic factors, such as Neurotrophin 3 (NTF3), epidermal growth factor (EGF), neurite growth-promoting factor 2 (NEGF2/MDK), heparin binding EGF-like growth factor (HBEGF), Chemokine ligand 2 (CXCL2), CXCL5, and fibroblast growth factor 9 (FGF9) [
195].
Angiogenesis/wound healing—Being mainly involved in WJ remodeling, UC-MSCs are not in contact with capillaries and small blood vessels, excluding the unique three vessels involved in umbilical blood circulation (the two arteries and the one vein), and they produce small amounts of angiogenic factor VEGF-A. Their support on endothelial cell proliferation and vasculogenesis could be related with other VEGF-independent factors, such as IL-8, hepatocyte growth factor (HGF), and MCP-1 [
196].
Matrix remodeling—The UC-MSCs are involved in ECM composition of WJ that surrounds the umbilical vessels, expressing vimentin and collagen II [
163]. Lo Iacono et al., demonstrated, using mass spectrometry analyses, that UC-MSCs co-cultured with umbilical cord blood–CD34+ hematopoietic stem/progenitor cells are able to secrete collagens, different proteases and their inhibitors, such as MMP-8, TIMP-1, ADAM with thrombospondin type 1 motif 9 (ADAMTS9), secreted protein acidic and cysteine rich (SPARC), plasminogen activator inhibitor-1 (PAI-1), and serine carboxypeptidase 1 involved in ECM remodeling, as well as α-2-HS glycoprotein, which is a TGF-β antagonist and prevents calcification by buffering excess matrix mineralization, in addition to than mimicking a hematopoietic niche [
158]. Migration of cells on ECM, remodeling, and degradation of the ECM by MMPs are key regulators of wound repair, since wound healing requires the controlled activity of MMPs, and UC-MSCs may have a great potential in connective tissues and wounds [
196,
197,
198].
Hypoxia resistance—Another aspect worthy of note about UC-MSCs is their high resistance to a stromal environment that is relatively hypoxic, therefore adapting to survive in limited nutrient and oxygen conditions [
199]. To this regard, scholars recently demonstrated similar metabolism and survival capability in both normal and hypoxic conditions (in oxygen–glucose deprivation/reperfusion stroke model) exerted by the three different MSC populations isolated from the three different zones of umbilical stromal, Wharton’s jelly (WJ-MSCs), perivascular region (PV-MSCs), and cord lining (CL-MSCs) of human, suggesting that UC-MSCs are suitable for stem-cell-based therapy of ischemic diseases [
200]. This provides the idea that tissue function support may be due to the transfer of healthy mitochondria [
201], which has been demonstrated, improving oxidative phosphorylation (OXPHOS) and bioenergetics of recipient cells [
202].
The paracrine effects of UC-MSCs-derived molecules with immunomodulatory, anti-inflammatory, and regenerative properties are related to their release by MSCs on the extracellular environment not only through the secretion of soluble factors, but also as cargos of EVs, such as exosomes, which also contain lipids, metabolites, DNA fragments, miRNA fragments, and noncoding RNAs, acting locally and/or at distance as a cell-to-cell communication system, both in physiological and pathological conditions [
203,
204,
205]. Due to their structure, and the opportunity to freely circulate into the body fluids, with low immunogenicity, and their bioavailability, they could be useful for drug delivery, and generate a great interest among scientists for cell-free therapies. The roles of perinatal MSCs and their conditioned media, enriched in EVs, were also explored in preventing lung injury across lung transplantation, described by Miceli and coworkers [
206,
207].
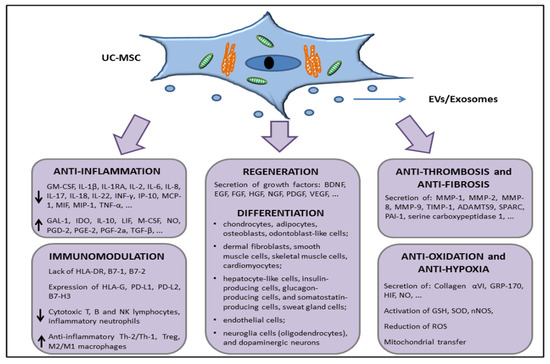
Taken together, all these features, characterized by the surface expression or secretion (through EVs/exosomes or in soluble form) of a series of factors with anti-inflammatory, immunomodulation, tissue repair, antifibrotic, and antihypoxic functions (summarized in Figure 3), support the interest in further studies about the use of UC-MSCs in in vivo experiments in order to move to allogeneic transplantation or develop novel cell-free products that are able to restore tissue functions, such as lung parenchymal functions dampening the damages exerted during COVID-19 disease.
Figure 3. Schematic representation of the UC-MSCs effects related with molecules involved in direct cell-to-cell interaction or through the release of extracellular vesicles (EVs) and/or exosomes.
2.3. In Vivo Preclinical Data Supporting the Use of UC-MSCs to Treat Organ Dysfunctions
The researchers worldwide have been focused on xenogeneic UC-MSCs in in vivo preclinical trials for determining safety, tolerance, and efficacy.
It was found that UC-MSCs transplantation induced, in vivo, a weak activation of immune Th1 and Th2 cells, and they showed significantly longer survival times in immunocompetent Balb/c mice compared to BM-MSCs, with a survival time prolonged in immunodeficient SCID-beige mice that must be attributed to the compromised immune response of the host [
208].
Transplantation by intravenous (IV) injection in female dark agouti rats, used for EAE model, revealed that UC-MSCs were detected in lung and spleen 2.5 weeks after transplantation in the chronic disease phase, but they were not observed in the lymph nodes, spinal cord, or brain of transplanted animals. However, they potently (even if transiently) ameliorated neurological symptoms [
209]. As explained above, multiple sclerosis could be alleviated by neuroprotective and neuroregenerating properties of LIF released in the bloodstream by UC-MSCs [
190].
In a myocardial infarction murine model, induced by left anterior descending (LAD) coronary artery ligation, delivery of UC-MSCs by intramyocardial injection resulted in a reduction of scar in the left ventricular wall thickness and stimulation of angiogenesis, preventing apoptosis and attenuating adverse tissue remodeling, compared to the vehicle control group [
211].
Although UC-MSCs do not differentiate into any lung cell, in the bleomycin-induced murine model of lung injury, these cells show homing to the lung tissue at 14 days after injection (but not at 28 days) compared to healthy mice, and showed antifibrotic properties increasing MMP-2, inducing the reduction of endogenous inhibitors (TIMP-1 to -4), and decreasing inflammation by repressing the expression of TGF-β, IFN-γ, and the proinflammatory cytokines macrophage migratory inhibitory factor (MIF) and TNF-α [
213].
Even UC-MSCs-derived EVs have been under the microscope for treating organ dysfunction. In fact, as reviewed by Lelek and Zuba-Surma, in vivo preclinical studies on EVs derived from UC-MSCs have been conducted in neurological, cardiovascular, liver, kidney, and skin diseases [
214]. In a preclinical model of bronchopulmonary dysplasia (BPD), using neonatal mice exposed to hyperoxia (75% O
2), the IV injection with UC-MSCs-derived exosomes restored lung morphology, postnatal development, pulmonary hypertension, and vascular remodeling, along with decreased lung fibrosis [
215].
3. MSCs in COVID-19 Patients
Based on just-reported evidence about the shorter survival time of BM-MSCs compared with UC-MSCs [
208], there is still the consensus about the use of BM-MSCs as a “gold-standard” for cell therapy. This is because it is relatively safer to use autologous BM-MSCs (or AT-MSCs) from the same patients, compared to allogeneic UC-MSCs, but the age of the patients, their gender, their health conditions, and the invasive procedure for isolating BM-MSCs (or AT-MSCs) must be taken into account in order to balance pros and cons. Even BM-MSCs have been studied in COVID-19 patients, as reviewed in Yao et al. [
217]. BM-MSCs are able to produce and secrete soluble PD-1 ligands (sPD-L1 and sPD-L2) that are responsible for hyporesponsiveness in T cells, arresting the PD-1-mediated AKT pathway, thus inducing immune tolerance [
218]. Further, in in vitro and in a humanized mouse model of graft versus host disease (GvHD), it was described that BM-MSCs affected T lymphocyte proliferation more than UC-MSCs, while the latter induced a higher increase of Tregs/Th17 ratio [
219]. Since Treg/Th17 ratio imbalance correlates with immune thrombocytopenia (ITP) [
220] and this imbalanced ratio was also observed in different cases of COVID-19 disease [
221], the reactions of infused MSCs for correcting immune response in such condition should be absolutely taken in consideration. For example, the patients with ITP displayed abnormalities in BM-MSCs, due to defects in mRNA and miRNA that induced downregulation of genes involved in cellular stress machinery, such as the unfolded protein response (UPR), the nuclear protein transcriptional regulator 1 (Nupr1), involved in endoplasmic reticulum pathway, the TGF-β1 signaling, leading to a loss of immunosuppressive properties, and a breakdown of self-tolerance in ITP patients [
222]. In this specific case, ITP patients are not eligible for autologous BM-MSCs.
In a pilot study involving liver allograft recipients with acute rejection, they also observed a significant increase of Treg/Th17 ratio after 4 weeks of UC-MSC infusion [
223]. Moreover, the BM-MSCs revealed a donor’s age-related decrease in colony forming units-fibroblast (CFU-f) in growth rate, in differentiation potential, and in superoxide dismutase (SOD) activity (and an increase of reactive oxygen species production) that could, in turn, affect autologous cell-based therapy [
224]. On the contrary, UC-MSCs derived from perinatal tissue of childbearing age population showed no sign of senescence over several passages [
157]. An integrated transcriptome-proteome analysis, comparing MSCs from different sources (BM, AT, and UC) revealed that secretome derived from UC-MSCs had a predominantly anti-inflammatory effect enriched in T cell inhibitory interleukins, such as IL-4, IL-13, IL-6, IL-35, IL-2, IL-22, IL-1R1, and IL-25, as well as the colony-stimulating growth factor (CSF) 3, which promoted M2 macrophage polarization, compared with the adult MSCs, while BM-MSCs were more immunosuppressive [
225]. The immunosuppression is probably initiated starting from the apoptotic events induced by cytotoxic cells against BM-MSCs infused in GvHD recipients, as a result of a bystander effect of CD56
+ natural killer (NK) and CD8
+ T cells [
226]. Taken together, these findings could highlight that UC-MSCs are more effective in treating symptoms and inflammatory state during the COVID-19-related cytokine storm than BM-MSCs.