Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Biomedical
The limited regenerative capacity of the human body, in conjunction with a shortage of healthy autologous tissue, has created an urgent need for alternative grafting materials. A potential solution is a tissue-engineered graft, a construct which supports and integrates with host tissue. One of the key challenges in fabricating a tissue-engineered graft is achieving mechanical compatibility with the graft site; a disparity in these properties can shape the behaviour of the surrounding native tissue, contributing to the likelihood of graft failure.
- scaffold
- biomimetic
- tissue engineering
- 3D printing
1. Introduction
The means of restoring mechanical functionality to damaged biological tissues has proven to be a longstanding challenge to medical practitioners. The complexity of the task is largely due to the intricacy of the native tissue, where biomechanical properties are largely dictated by the extracellular matrix (ECM) [1][2]. This structure consists of a multifaceted network of various constituents such as water, polysaccharides, and proteins such as collagen and elastin [3]. When under load, the ECM relies primarily on these proteins working in conjunction with one another for support [4], with the collagen providing mechanical resistance to deformation under increased load conditions, and elastin being responsible for maintaining the elasticity of the tissue and resistance under relatively low loading [5]. Mechanical integrity and elasticity are not only critical for explicit functions such as joint articulation and other musculoskeletal interactions; they are just as significant for various parasympathetic processes such as peristalsis, respiration, and vasodilation [6][7][8]. The degradation of tissue biomechanics, whether via natural processes such as ageing, or via exposure to circumstances leading to internal or external injury of soft and hard tissues, can precede issues ranging from loss of mobility and discomfort, to critical conditions such as pulmonary fibrosis and supravalvular aortic stenosis [9][10][11].
The complexity of repairing extensively damaged tissues is such that contemporary regenerative medicinal practices are limited in treatment options [12]. The transplantation of fresh tissue is seen as the optimal solution, as the new material is theoretically able to fulfil the physiological and mechanical requirements of the original tissue [13]. However, both allografts and autologous grafts bear several limitations. In the case of allografting, graft-versus-host disease, transplant rejection, bleeding, and infection are constant risk factors [14], whereas autologous grafting is not always an option, due to either previous harvesting of the donor site or systemic disease rendering the tissue unsuitable [15]. An alternative therapeutic in promoting the repair of damaged tissues, stem cell treatment, is largely in its infancy as a research area, and faces several significant hurdles, namely, a high risk of immune response, scalability, and overcoming the negative public perception of such a treatment [16][17]. An alternative means of restoring the mechanical properties of biological tissue is therefore necessary in order to address the ever-increasing demand for biological tissue transplantation.
Tissue engineering is a rapidly expanding field which aims to design constructs which supplement or replace damaged biological tissue. The fabrication of a tissue-engineered graft can be undertaken in a variety of ways, including phase separation, bioprinting, gas foaming and electrospinning techniques [18][19][20][21][22][23]. From these methods, a dense interconnected network of material may be formed, which is termed a scaffold [24]. The purpose of a scaffold is to provide a chemically and mechanically appropriate environment to facilitate cellular growth, which may then be implanted into a given patient [25][26][27]. The ideal tissue-engineered scaffold would allow the development and restoration of fully functional tissue at the graft site, maintaining structural integrity under physiological stress, and ultimately be removed by the body’s natural processes after fulfilling its function [28][29][30].
A key challenge within this area is ensuring the greatest possible resemblance between the mechanical response of the tissue-engineered graft and the host’s native tissue. This is a crucial aspect of a successful design, as biological tissues are load-bearing by nature, regardless of function, from the strongest regions of cortical bone with mechanical strength in the gigapascal range [31] to the most delicate of neural fibres throughout the nervous system whose strength generally lies within the low kilopascal scale [32]. The requirement to maintain the physical integrity of the scaffold while also retaining biocompatibility has given rise to so-called ‘biomaterials’, which the majority of tissue-engineered scaffolds are composed of and which fall into several broad camps: natural polymers, synthetic polymers, ceramics, decellularized matrices, hydrogels, and metals [33][34][35][36][37][38]. Natural polymers can include agarose, alginates, and chitosan while synthetic polymers include polycaprolactone, poly(l-lactic acid), and Poly(ethylene glycol) diacrylate. Ceramics can comprise aluminium oxide, bioglass, and hydroxyapatite, while decellurised matrices can be derived from almost any bodily feature, such as bones and organs. Hydrogels are typically composed of naturally derived constituents such as collagen, gelatine and hyaluronic acid, and metals used for scaffolds can include magnesium, tantalum, and titanium [26][36][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53]. The broad range of characteristics of these materials allows for a vast range of tissue types to be mechanically accounted for; an overview of the typical strength of these material groups in comparison to the stiffness of organic tissues is given in Figure 1 [32][54][55][56][57][58][59][60][61][62][63][64][65][66][67].
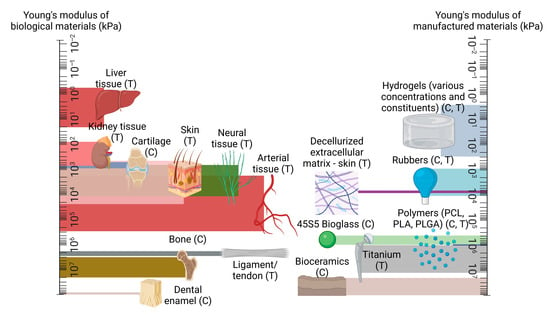
Figure 1. A comparison of mechanical strength between native tissue types, in comparison to manufactured materials, presented on logarithmic scales, with compression and tension testing denoted with (C) and (T) as appropriate. Created using Biorender.com, 15 May 2023.
2. Mechanical Properties of Biological Tissues and Their Influence
The mechanical properties of biological materials are a product of a complex array of proteins, cells, and interstitial fluid flow, whose interactions characterise the response of their respective tissue under load [68][69]. As a product of their intricacy, biological tissues exhibit complex mechanical properties such as heterogeneity, viscoelasticity, and anisotropy [68][69][70]. Accurately assessing the mechanical properties of these tissues is therefore intrinsically challenging, with research groups often attaining a variety of results for the same tissue.
The diversity in these results can not only be attributed to the structural composition of the tissues alone; factors such as hydration and age are also known to affect the mechanical response of biological tissues [71][72]. This process of tissue-specific cells responding to mechanical stimuli is termed mechanotransduction, which is a means by which organic tissue may convert external loading, such as tension or vibrational waves, to biochemical, biophysical, or molecular signals via a series of events culminating in a cellular response [73]. Figure 2 illustrates this process, beginning with a given external loading event encountered by the body’s mechanosensors. This is termed mechanocoupling, and, depending on the load type, is translated by one or more mechanosensors to adjacent cells in the ECM [74]. Via a complex series of intra-cellular interactions, these mechanical signals are converted to various signal types which instruct the relevant cell groups to behave in a particular way; for example, an increase in mechanical load due to extensive physical activity can lead to compensatory growth in relevant skeletal and muscular members [73][75].
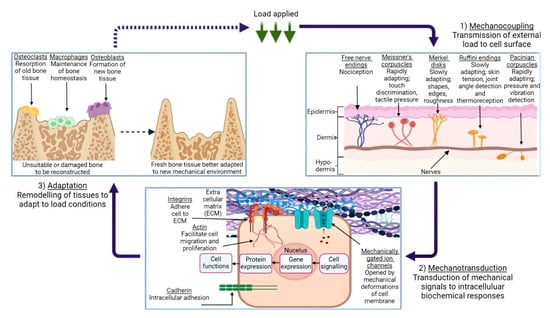
Figure 2. An illustration of the mechanotransduction cycle, in the context of osseous tissue remodelling in response to external loading applied to a given area of skin, via the medium of a generic cuboidal cell. (1) demonstrates the various types of mechanoreceptors present in human dermis and their function, while (2) depicts the mechanotransduction pathway, followed when the ECM is subject to deformation. (3) shows the remodelling process of bone tissue in response to this loading, and the roles which the various cells play in this process. Created using BioRender.com, 24 April 2023.
It is the cellular response to various conditions which allows the human body to maintain homeostasis, while also quickly adapting to environmental circumstances; vasoconstriction and vasodilation are common examples of this [76]. However, a crucial caveat is that biological tissues have evolved to expect a certain degree of physiological loading. A reduction in the stress and strain forces imparted on, for example, the head region of the femur as a result of a hip implant, can in turn lead to the ‘stress-shielding’ effect [77]. This is where a non-native element acts as the main load-bearing facilitator during typical movement (e.g., walking or running). As a result, the incentive for cells to remodel and strengthen their respective tissues during mechanotransduction is lowered and local bone density is reduced, potentially leading to implant loosening, stress fracturing, and abnormal bone development [78][79].
3. Hybrid Materials
The non-linear biomechanical response of a tissue under load is difficult to reproduce in synthetic materials alone, as this response is characterised by complex biological structures and interactions, as described in Section 2. Current research indicates that a hybrid polymer blend, consisting of both synthetic and natural materials, allows for constructs which better represent this intricate behaviour [80]. This is due to the naturally derived constituent typically retaining its microstructural details, which can promote cell attachment and integration; this is beneficial when employing a scaffold in a biological environment [81][82]. A broad range of hybrid polymer combinations are currently undergoing extensive research, ranging from binary PCL-hydroxyapatite designs [29] to complex three- or four-part hybrid amalgamations [83][84].
The scope for hybrid biomaterial design is vast, which allows researchers to consider a broad range of materials. For example, several research groups have recently investigated the inclusion of multi-walled carbon nanotubes (MWCNTs), which are nanometre-diameter carbon-based tubular constructs, with the aim of utilising the nanotubes’ high tensile strength and electrical/thermal conductivity to enhance the overall biomechanical properties of their scaffold. Sang et al. dispersed MWCNTs with concentrations ranging between 1, 3, and 5% through a chitosan/polyethylene glycol scaffold, which was prepared by freeze-drying, for the purpose of neural tissue engineering [85]. The electrical conductivity of neurons is key to their function, and one of the key findings of this analysis was the rise in electrical conductivity of the scaffold in proportion to increasing MWCNT concentrations. Interestingly, the elastic modulus of the hybrid scaffold was also enhanced with an increasing concentration of MWCNTs, from 1.17 kPa to 2.09 kPa, demonstrating the load-bearing abilities of carbon nanotubes.
Hybrid plant-based designs are also promising in this area; a recent study by Jiawei et al. investigated the inclusion of hydroxyapatite into sugarcane stem, whose lignin had been removed (i.e., delignified) via soaking in a NaClO2/acetate buffer solution. The degree of delignification induced a proportionate increase in the sugarcane stem porosity, albeit at the cost mechanical performance under compressive load, with the greatest extent—8 h of immersion—reducing the compressive modulus of 14.6 MPa for pure sugarcane stem to 4.82 MPa.
4. Multi-Layer Scaffolds
The challenge of tuning the elastic response of a scaffold in tandem with balancing its biocompatibility characteristics has led several research groups to fabricate scaffolds consisting of multiple layers. This approach allows for the functionalisation of each stratum independently and more closely represents the layered nature of most biological tissue, such as arterial walls, osseous tissue, and oesophageal tissue [86][87][88]. The potential impact of various material and fabrication combinations has allowed researchers to explore a broad range of designs consisting of materials such as silk, graphene, and titanium [89][90][91].
While the properties of multi-layered scaffolds are largely determined by the material selection, several groups have investigated the potential of applying novel fabrication methods in order to construct a multi-layered construct which can function within the physiological load range. Killian et al. studied the combination of calcium phosphate cement (CPC), fabricated via 3D printing, and PCL, drawn using a melting electrowriting (MEW) process for the purpose of bone tissue engineering [92]. These two materials were printed upon one another up to 10 layers in height, creating a grid-like pattern of CPC which contained multiple interspatial variations, and from which the strands of PCL fibre could intersect. Interestingly, while the addition of the PCL fibres slightly diminished the yield strength and elastic modulus of the scaffolds (which were approximately 4–10 and 40–85 MPa, respectively), the inclusion of these fibres would occasionally cause the construct to mechanically yield twice under load: once for the PCL strands, and again as the CPC failed, offering an element of redundancy to the design.
In addition, Thompson et al. employed a process known as embedded 3D printing to fabricate a multi-layer scaffold in an effort to replicate human vocal cord tissue [93]. This method utilises a cavity which has pre-printed ink filaments embedded within a given medium, allowing for the fabrication of a component without interference from gravity or requiring additional supports [94].
5. Surface Modification
The mechanical properties of engineered tissue are not necessarily set in stone. By design, a substance such as a polymeric compound may have its constituents altered such that it may better serve its intended function [95][96]. In a similar manner, a tissue-engineered graft, being typically composed of one or more complex substances, may be modified in such a way that a desired property, such as increased mechanical strength, can be achieved without having to resort to less suitable or more expensive material alternatives, via processes such as annealing or salt leaching [97][98]. Recent studies have considered a broad range of surface modifications, depending on the desired functionality of the design. These range from topographical nano-modification of the scaffold [99] to grafting post-fabrication and polymer coatings [100][101].
Surface modification also has the capacity to facilitate the functionalisation of more unlikely material candidates for this research into more suitable substances. An example of this is offered by Mahendiran et al., who examined the potential of cellulose scaffolds derived from Borassus flabellifer for the purpose of bone tissue regeneration [102]. The scaffolds were derived from the plant’s immature endosperm which was then, after washing and oxidising the scaffolds, modified by two organosilanes; amino-terminated aminopropyltriethoxysilane (APTES) and methyl-terminated octadecyltrichlorosilane (OTS). The subsequent scaffolds formed a foamy architecture, and, in comparison to the unmodified constructs employed as a control during the study, both the APTES and OTS treatments enhanced the compressive modulus of the material, from approximately 0.3 MPa to 0.9 MPa and 1.2 MPa, respectively. Crucially, both treatment methods also bore osteoinductive properties, demonstrating the potential of such a design in a clinical context.
Nanotopographical roughness is a feature which may also be employed to modify the properties of a given scaffold. The influence of surface roughness on cell behaviour is well documented, with several groups reporting relative increases in cell proliferation, adhesion, and desired protein expression when seeded on irregular scaffold surface morphologies [103][104][105]. One method of creating roughness on engineered scaffolds is alkaline hydrolysis, which was employed by Meng et al. to alter an existing PLLA design for the purposes of bone tissue engineering [106]. For this particular study, the scaffolds, formed via MEW, were immersed in alkali solutions consisting of concentrations of 0.25 M and 0.5 M sodium hydroxide (NaOH) in ethanol (1:1 ratio) for 1, 2, 3, and 4 h at a time. While the effect of this process was perhaps most notable in terms of cell count, which, after 15 days, was largely increased in comparison to the control scaffold, perhaps the most surprising result was the improved tensile modulus of the 0.5 M NaOH scaffold. After 1, 2, and 3 h periods of immersion, a maximum modulus of 5 GPa was achieved, in comparison to 2.5 GPa of the control scaffold. It was theorised that the alkaline treatment process increased the crystallinity of the individual PLLA fibres, thus providing additional tensile strength for the scaffold overall.
6. In Vitro Limitations and Animal Research
A construct designed to operate in such an environment, such as a vascular graft, would be expected to function under these load conditions; mechanical testing is therefore key, prior to the further development of such a design.
The complex nature of these forces has led scientists to explore several avenues of research in an effort to better comprehend, and design for, their impact on tissue-engineered designs. Recent advances in finite element analysis (FEA) and computational fluid dynamics (CFD) analyses have allowed researchers to model tissues such as pulmonary arteries [107], airways [108], ventricles [109], and eye lenses [110], which carries benefits ranging from a greater understanding of the morphological and physiological characteristics of these tissue types, to assisting surgeons during complex surgical procedures [111][112].
Despite the promising outlook of this research, a true estimation of the in vivo performance of a tissue-engineered scaffold is still beyond the grasp of contemporary computational or in vitro methods.
The next logical step, therefore, is to assess the viability of tissue-engineered implants in vivo. This will allow researchers to understand how viable their scaffold’s design is once implanted into biological tissue, and will also validate the use of particular materials and construction methods within living organisms.
The assessment of tissue-engineered designs in vivo can also offer insight into cell behaviour when uncommon scaffold morphologies are implanted within the animal. Feng et al. examined the effects of implanting a conch-like scaffold, featuring a helical inner structure, into the upper femoral region of New Zealand rabbits, with the aim of assessing such a design to promote guided bone regeneration [113]. Formed via 3D printing with β-tricalcium phosphate, various diameters, pitches, and pore sizes of the scaffold were examined, with a maximum compressive modulus of 1.75 GPa achieved with a 9.8 mm scaffold diameter, 1 mm pore diameter, and 2.4 mm pitch. It was found that the distinctive design of the scaffold encouraged a capillary action effect when placed within a cell media solution; this phenomenon was fully demonstrated when the scaffold was assessed in vivo, as the cells rapidly proliferated up the helical section, illustrating the directional osteoinductive benefits of such a scaffold morphology.
This entry is adapted from the peer-reviewed paper 10.3390/biomimetics8020205
References
- Sarasa-Renedo, A.; Chiquet, M. Mechanical signals regulating extracellular matrix gene expression in fibroblasts. Scand. J. Med. Sci. Sports 2005, 15, 223–230.
- Papanicolaou, M.; Cox, T.R. Extracellular Matrix (ECM). In Encyclopedia of Molecular Pharmacology; Springer: Cham, Switzerland, 2022; pp. 643–650.
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma 2014, 23 (Suppl. 1), S20–S23.
- Chow, M.-J.; Turcotte, R.; Lin, C.P.; Zhang, Y. Arterial Extracellular Matrix: A Mechanobiological Study of the Contributions and Interactions of Elastin and Collagen. Biophys. J. 2014, 106, 2684–2692.
- Baumann, L.; Bernstein, E.F.; Weiss, A.S.; Bates, D.; Humphrey, S.; Silberberg, M.; Daniels, R. Clinical Relevance of Elastin in the Structure and Function of Skin. Aesthetic Surg. J. Open Forum 2021, 3, ojab019.
- Sinnott, M.D.; Cleary, P.W.; Harrison, S.M. Peristaltic transport of a particulate suspension in the small intestine. Appl. Math. Model. 2017, 44, 143–159.
- Mecham, R. Elastin in lung development and disease pathogenesis. Matrix Biol. 2018, 73, 6–20.
- Faury, G.; Ristori, M.; Verdetti, J.; Jacob, M.; Robert, L. Effect of Elastin Peptides on Vascular Tone. J. Vasc. Res. 1995, 32, 112–119.
- Godinho, M.S.C.; Thorpe, C.T.; Greenwald, S.E.; Screen, H.R.C. Elastin is Localised to the Interfascicular Matrix of Energy Storing Tendons and Becomes Increasingly Disorganised with Ageing. Sci. Rep. 2017, 7, 9713.
- De Brouwer, B.; Drent, M.; van den Ouweland, J.M.; Wijnen, P.A.; van Moorsel, C.H.; Bekers, O.; Grutters, J.C.; White, E.S.; Janssen, R. Increased circulating desmosine and age-dependent elastinolysis in idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 45.
- Merla, G.; Brunetti-Pierri, N.; Piccolo, P.; Micale, L.; Loviglio, M.N. Supravalvular aortic stenosis: Elastin arteriopathy. Circ. Cardiovasc. Genet. 2012, 5, 692–696.
- Heinz, A. Elastic fibers during aging and disease. Ageing Res. Rev. 2021, 66, 101255.
- Rouchi, A.H.; Mahdavi-Mazdeh, M. Regenerative Medicine in Organ and Tissue Transplantation: Shortly and Practically Achievable? Int. J. Organ Transplant. Med. 2015, 6, 93–98.
- Chiarello, E.; Cadossi, M.; Tedesco, G.; Capra, P.; Calamelli, C.; Shehu, A.; Giannini, S. Autograft, allograft and bone substitutes in reconstructive orthopedic surgery. Aging Clin. Exp. Res. 2013, 25, 101–103.
- Tonk, G.; Yadav, P.K.; Agarwal, S.; Jamoh, K. Donor site morbidity in autologous bone grafting—A comparison between different techniques of anterior iliac crest bone harvesting: A prospective study. J. Orthop. Trauma Rehabil. 2022, 29, 22104917221092163.
- Batten, P.; Rosenthal, N.; Yacoub, M.H. Immune response to stem cells and strategies to induce tolerance. Philos. Trans. R. Soc. B: Biol. Sci. 2007, 362, 1343–1356.
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68.
- Heim, M.; Nixon, I.J.; Emmerson, E.; Callanan, A. From hormone replacement therapy to regenerative scaffolds: A review of current and novel primary hypothyroidism therapeutics. Front. Endocrinol. 2022, 13, 2392.
- Handley, E.; Callanan, A. Modulation of Tissue Microenvironment following Myocardial Infarction. Adv. NanoBiomed Res. 2022, 2, 2200005.
- Sturtivant, A.; Callanan, A. The use of antifreeze proteins to modify pore structure in directionally frozen alginate sponges for cartilage tissue engineering. Biomed. Phys. Eng. Express 2020, 6, 055016.
- Sofokleous, P.; Chin, M.H.; Day, R. Phase-separation technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds—Materials, Technologies and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 101–126.
- Xie, Z.; Gao, M.; Lobo, A.O.; Webster, T.J. 3D Bioprinting in Tissue Engineering for Medical Applications: The Classic and the Hybrid. Polymers 2020, 12, 1717.
- Costantini, M.; Barbetta, A. Gas foaming technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Woodhead Publishing: Sawston, UK, 2018; pp. 127–149.
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. 4), 467–479.
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427.
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823.
- Hosseinkhani, M.; Mehrabani, D.; Karimfar, M.H.; Bakhtiyari, S.; Manafi, A.; Shirazi, R. Tissue Engineered Scaffolds in Regenerative Medicine. World J. Plast. Surg. 2014, 3, 3–7.
- Qiu, Y.; Chen, X.; Hou, Y.; Hou, Y.; Tian, S.; Chen, Y.; Yu, L.; Nie, M.; Liu, X. Characterization of different biodegradable scaffolds in tissue engineering. Mol. Med. Rep. 2019, 19, 4043–4056.
- Rezania, N.; Asadi-Eydivand, M.; Abolfathi, N.; Bonakdar, S.; Mehrjoo, M.; Solati-Hashjin, M. Three-dimensional printing of polycaprolactone/hydroxyapatite bone tissue engineering scaffolds mechanical properties and biological behavior. J. Mater. Sci. Mater. Med. 2022, 33, 31.
- De Pieri, A.; Rochev, Y.; Zeugolis, D.I. Scaffold-free cell-based tissue engineering therapies: Advances, shortfalls and forecast. Npj Regen. Med. 2021, 6, 18.
- Jeong, W.-S.; Kim, Y.-C.; Min, J.-C.; Park, H.-J.; Lee, E.-J.; Shim, J.-H.; Choi, J.-W. Clinical Application of 3D-Printed Patient-Specific Polycaprolactone/Beta Tricalcium Phosphate Scaffold for Complex Zygomatico-Maxillary Defects. Polymers 2022, 14, 740.
- Borschel, G.H.; Kia, K.F.; Kuzon, W.M.; Dennis, R.G. Mechanical properties of acellular peripheral nerve. J. Surg. Res. 2003, 114, 133–139.
- Tomlins, P. 1—Material Types for Tissue Scaffolds, in Characterisation and Design of Tissue Scaffolds; Tomlins, P., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 1–21.
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Polymer-Based Scaffolds for Soft-Tissue Engineering. Polymers 2020, 12, 1566.
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202.
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2021, 10, 15–31.
- Radulescu, D.M.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. New Insights of Scaffolds Based on Hydrogels in Tissue Engineering. Polymers 2022, 14, 799.
- Koushik, T.M.; Miller, C.M.; Antunes, E. Bone Tissue Engineering Scaffolds: Function of Multi-Material Hierarchically Structured Scaffolds. Adv. Healthc. Mater. 2023, 12, 2202766.
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers 2020, 12, 1150.
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2020, 16, 280–306.
- Tian, Y.; Wu, D.; Wu, D.; Cui, Y.; Ren, G.; Wang, Y.; Wang, J.; Peng, C. Chitosan-Based Biomaterial Scaffolds for the Repair of Infected Bone Defects. Front. Bioeng. Biotechnol. 2022, 10, 755.
- Gao, Y.; Callanan, A. Influence of surface topography on PCL electrospun scaffolds for liver tissue engineering. J. Mater. Chem. B 2021, 9, 8081–8093.
- Dai, Y.; Lu, T.; Shao, M.; Lyu, F. Recent advances in PLLA-based biomaterial scaffolds for neural tissue engineering: Fabrication, modification, and applications. Front. Bioeng. Biotechnol. 2022, 10, 1011783.
- Kotturi, H.; Abuabed, A.; Zafar, H.; Sawyer, E.; Pallipparambil, B.; Jamadagni, H.; Khandaker, M. Evaluation of Polyethylene Glycol Diacrylate-Polycaprolactone Scaffolds for Tissue Engineering Applications. J. Funct. Biomater. 2017, 8, 39.
- Ammarullah, M.I.; Hartono, R.; Supriyono, T.; Santoso, G.; Sugiharto, S.; Permana, M.S. Polycrystalline Diamond as a Potential Material for the Hard-on-Hard Bearing of Total Hip Prosthesis: Von Mises Stress Analysis. Biomedicines 2023, 11, 951.
- Mazzoni, E.; Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Maritati, M.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Bioactive Materials for Soft Tissue Repair. Front. Bioeng. Biotechnol. 2021, 9, 61378.
- Bernardo, M.P.; da Silva, B.C.R.; Hamouda, A.E.I.; de Toledo, M.A.S.; Schalla, C.; Rütten, S.; Goetzke, R.; Mattoso, L.H.C.; Zenke, M.; Sechi, A. PLA/Hydroxyapatite scaffolds exhibit in vitro immunological inertness and promote robust osteogenic differentiation of human mesenchymal stem cells without osteogenic stimuli. Sci. Rep. 2022, 12, 2333.
- Amirazad, H.; Dadashpour, M.; Zarghami, N. Application of decellularized bone matrix as a bioscaffold in bone tissue engineering. J. Biol. Eng. 2022, 16, 1.
- Sarrigiannidis, S.O.; Rey, J.; Dobre, O.; González-García, C.; Dalby, M.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098.
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers 2023, 15, 1026.
- Chircov, C.; Grumezescu, A.M.; Bejenaru, L.E. Hyaluronic acid-based scaffolds for tissue engineering. Rom. J. Morphol. Embryol. 2018, 59, 71–76.
- Gao, H.; Yang, J.; Jin, X.; Qu, X.; Zhang, F.; Zhang, D.; Chen, H.; Wei, H.; Zhang, S.; Jia, W.; et al. Porous tantalum scaffolds: Fabrication, structure, properties, and orthopedic applications. Mater. Des. 2021, 210, 110095.
- Hanawa, T. Titanium–Tissue Interface Reaction and Its Control With Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170.
- Sprague, A.L.; Awokuse, D.; Pohlig, R.T.; Cortes, D.H.; Silbernagel, K.G. Relationship between mechanical properties (shear modulus and viscosity), age, and sex in uninjured Achilles tendons. Transl. Sports Med. 2020, 3, 321–327.
- LaCroix, A.S.; Duenwald-Kuehl, S.E.; Lakes, R.S.; Vanderby, R. Relationship between tendon stiffness and failure: A metaanalysis. J. Appl. Physiol. 2013, 115, 43–51.
- Maganaris, C.N.; Paul, J.P. In vivo human tendon mechanical properties. J. Physiol. 1999, 521 Pt 1, 307–313.
- Urban, M.W.; Rule, A.D.; Atwell, T.D.; Chen, S. Novel Uses of Ultrasound to Assess Kidney Mechanical Properties. Kidney360 2021, 2, 1531–1539.
- Karimi, A.; Shojaei, A. Measurement of the Mechanical Properties of the Human Kidney. IRBM 2017, 38, 292–297.
- Palmeri, M.L.; Wang, M.; Dahl, J.; Frinkley, K.; Nightingale, K. Quantifying Hepatic Shear Modulus In Vivo Using Acoustic Radiation Force. Ultrasound Med. Biol. 2008, 34, 546–558.
- Kemper, A.R.; Santago, A.C.; Stitzel, J.D.; Sparks, J.L.; Duma, S.M. Biomechanical response of human liver in tensile loading. Ann. Adv. Automot. Med. 2010, 54, 15–26.
- Yeh, W.-C.; Li, P.-C.; Jeng, Y.-M.; Hsu, H.-C.; Kuo, P.-L.; Li, M.-L.; Yang, P.-M.; Lee, P.H. Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med. Biol. 2002, 28, 467–474.
- Arikawa, H. Dynamic Shear Modulus in Torsion of Human Dentin and Enamel. Dent. Mater. J. 1989, 8, 223–235,287.
- Chun, K.; Choi, H.; Lee, J. Comparison of mechanical property and role between enamel and dentin in the human teeth. J. Dent. Biomech. 2014, 5, 1758736014520809.
- Aghajanian, A.H.; Bigham, A.; Sanati, A.; Kefayat, A.; Salamat, M.R.; Sattary, M.; Rafienia, M. A 3D macroporous and magnetic Mg2SiO4-CuFe2O4 scaffold for bone tissue regeneration: Surface modification, in vitro and in vivo studies. Biomater. Adv. 2022, 137, 212809.
- Park, S.; Tao, J.; Sun, L.; Fan, C.-M.; Chen, Y. An Economic, Modular, and Portable Skin Viscoelasticity Measurement Device for In Situ Longitudinal Studies. Molecules 2019, 24, 907.
- Gauthier, R.; Attik, N.; Chevalier, C.; Salles, V.; Grosgogeat, B.; Gritsch, K.; Trunfio-Sfarghiu, A.-M. 3D Electrospun Polycaprolactone Scaffolds to Assess Human Periodontal Ligament Cells Mechanobiological Behaviour. Biomimetics 2023, 8, 108.
- Mirzaali, M.J.; Schwiedrzik, J.J.; Thaiwichai, S.; Best, J.P.; Michler, J.; Zysset, P.K.; Wolfram, U. Mechanical properties of cortical bone and their relationships with age, gender, composition and microindentation properties in the elderly. Bone 2016, 93, 196–211.
- Gough, A.; Stern, A.M.; Maier, J.; Lezon, T.; Shun, T.-Y.; Chennubhotla, C.; Schurdak, M.E.; Haney, S.A.; Taylor, D.L. Biologically Relevant Heterogeneity: Metrics and Practical Insights. SLAS Discov. Adv. Sci. Drug Discov. 2017, 22, 213–237.
- Zhang, K.; Zhu, M.; Thomas, E.; Hopyan, S.; Sun, Y. Existing and Potential Applications of Elastography for Measuring the Viscoelasticity of Biological Tissues In Vivo. Front. Phys. 2021, 9, 670571.
- Mitchell, G.R.; Tojeira, A. Role of Anisotropy in Tissue Engineering. Procedia Eng. 2013, 59, 117–125.
- Nicolle, S.; Palierne, J.-F. Dehydration effect on the mechanical behaviour of biological soft tissues: Observations on kidney tissues. J. Mech. Behav. Biomed. Mater. 2010, 3, 630–635.
- Phillip, J.M.; Aifuwa, I.; Walston, J.; Wirtz, D. The Mechanobiology of Aging. Annu. Rev. Biomed. Eng. 2016, 17, 113–141.
- Martino, F.; Perestrelo, A.R.; Vinarsky, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824.
- Mierke, C.T.; Kollmannsberger, P.; Zitterbart, D.P.; Smith, J.; Fabry, B.; Goldmann, W.H. Mechano-Coupling and Regulation of Contractility by the Vinculin Tail Domain. Biophys. J. 2008, 94, 661–670.
- Khan, K.M.; Scott, A. Mechanotherapy: How physical therapists’ prescription of exercise promotes tissue repair. Br. J. Sports Med. 2009, 43, 247–252.
- Charkoudian, N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J. Appl. Physiol. 2010, 109, 1221–1228.
- Yamako, G.; Janssen, D.; Hanada, S.; Anijs, T.; Ochiai, K.; Totoribe, K.; Chosa, E.; Verdonschot, N. Improving stress shielding following total hip arthroplasty by using a femoral stem made of β type Ti-33.6Nb-4Sn with a Young’s modulus gradation. J. Biomech. 2017, 63, 135–143.
- Be’Ery-Lipperman, M.; Gefen, A. A method of quantification of stress shielding in the proximal femur using hierarchical computational modeling. Comput. Methods Biomech. Biomed. Eng. 2007, 9, 35–44.
- Lei, B.; Guo, B.; Rambhia, K.J.; Ma, P.X. Hybrid polymer biomaterials for bone tissue regeneration. Front. Med. 2018, 13, 189–201.
- Wang, X.; Li, S.; Yu, H.; Lv, J.; Fan, M.; Wang, X.; Wang, X.; Liang, Y.; Mao, L.; Zhao, Z. The Biocompatibility of Multi-Source Stem Cells and Gelatin-Carboxymethyl Chitosan-Sodium Alginate Hybrid Biomaterials. Tissue Eng. Regen. Med. 2022, 19, 491–503.
- Nokhbatolfoghahaei, H.; Paknejad, Z.; Bohlouli, M.; Rad, M.R.; Aminishakib, P.; Derakhshan, S.; Amirabad, L.M.; Nadjmi, N.; Khojasteh, A. Fabrication of Decellularized Engineered Extracellular Matrix through Bioreactor-Based Environment for Bone Tissue Engineering. ACS Omega 2020, 5, 31943–31956.
- Nguyen, T.T.; Hu, C.C.; Sakthivel, R.; Nabilla, S.C.; Huang, Y.W.; Yu, J.; Cheng, N.C.; Kuo, Y.J.; Chung, R.J. Preparation of gamma poly-glutamic acid/hydroxyapatite/collagen composite as the 3D-printing scaffold for bone tissue engineering. Biomater. Res. 2022, 26, 21.
- Singh, B.N.; Nallakumarasamy, A.; Sinha, S.; Rastogi, A.; Mallick, S.P.; Divakar, S.; Srivastava, P. Generation of hybrid tissue engineered construct through embedding autologous chondrocyte loaded platelet rich plasma/alginate based hydrogel in porous scaffold for cartilage regeneration. Int. J. Biol. Macromol. 2022, 203, 389–405.
- Lee, J.U.; Kim, D.; Jang, C.H.; Kim, G.H. Highly elastic 3D-printed gelatin/HA/placental-extract scaffolds for bone tissue engineering. Theranostics 2022, 12, 4051–4066.
- Sang, S.; Cheng, R.; Cao, Y.; Yan, Y.; Shen, Z.; Zhao, Y.; Han, Y. Biocompatible chitosan/polyethylene glycol/multi-walled carbon nanotube composite scaffolds for neural tissue engineering. J. Zhejiang Univ. B 2022, 23, 58–73.
- Karšaj, I.; Humphrey, J.D. A multilayered wall model of arterial growth and remodeling. Mech. Mater. 2013, 44, 110–119.
- Liu, Y.; Luo, D.; Wang, T. Hierarchical Structures of Bone and Bioinspired Bone Tissue Engineering. Small 2016, 12, 4611–4632.
- Totonelli, G.; Maghsoudlou, P.; Fishman, J.M.; Orlando, G.; Ansari, T.; Sibbons, P.; A Birchall, M.; Pierro, A.; Eaton, S.; De Coppi, P. Esophageal tissue engineering: A new approach for esophageal replacement. World J. Gastroenterol. 2012, 18, 6900–6907.
- Wu, X.; Zhou, M.; Jiang, F.; Yin, S.; Lin, S.; Yang, G.; Lu, Y.; Zhang, W.; Jiang, X. Marginal sealing around integral bilayer scaffolds for repairing osteochondral defects based on photocurable silk hydrogels. Bioact. Mater. 2021, 6, 3976–3986.
- Dargoush, S.A.; Hanaee-Ahvaz, H.; Irani, S.; Soleimani, M.; Khatami, S.M.; Sohi, A.N. A composite bilayer scaffold functionalized for osteochondral tissue regeneration in rat animal model. J. Tissue Eng. Regen. Med. 2022, 16, 559–574.
- Yang, T.; Tamaddon, M.; Jiang, L.; Wang, J.; Liu, Z.; Liu, Z.; Meng, H.; Hu, Y.; Gao, J.; Yang, X.; et al. Bilayered scaffold with 3D printed stiff subchondral bony compartment to provide constant mechanical support for long-term cartilage regeneration. J. Orthop. Transl. 2021, 30, 112–121.
- Kilian, D.; von Witzleben, M.; Lanaro, M.; Wong, C.S.; Vater, C.; Lode, A.; Allenby, M.C.; Woodruff, M.A.; Gelinsky, M. 3D Plotting of Calcium Phosphate Cement and Melt Electrowriting of Polycaprolactone Microfibers in One Scaffold: A Hybrid Additive Manufacturing Process. J. Funct. Biomater. 2022, 13, 75.
- Zhao, J.; He, N. A mini-review of embedded 3D printing: Supporting media and strategies. J. Mater. Chem. B 2020, 8, 10474–10486.
- Greenwood, T.E.; Thomson, S.L. Embedded 3D printing of multi-layer, self-oscillating vocal fold models. J. Biomech. 2021, 121, 110388.
- Peng, Y.-Y.; Srinivas, S.; Narain, R. Chapter 5—Modification of Polymers, in Polymer Science and Nanotechnology; Narain, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 95–104.
- Sengupta, P.; Prasad, B.L.V. Surface Modification of Polymers for Tissue Engineering Applications: Arginine Acts as a Sticky Protein Equivalent for Viable Cell Accommodation. ACS Omega 2018, 3, 4242–4251.
- Browe, D.C.; Díaz-Payno, P.J.; Freeman, F.E.; Schipani, R.; Burdis, R.; Ahern, D.P.; Nulty, J.M.; Guler, S.; Randall, L.D.; Buckley, C.T.; et al. Bilayered extracellular matrix derived scaffolds with anisotropic pore architecture guide tissue organization during osteochondral defect repair. Acta Biomater. 2022, 143, 266–281.
- DiCerbo, M.; Benmassaoud, M.M.; Vega, S.L. Porous Scaffold-Hydrogel Composites Spatially Regulate 3D Cellular Mechanosensing. Front. Med. Technol. 2022, 4, 884314.
- Zhan, L.; Wang, L.; Deng, J.; Zheng, Y.; Ke, Q.; Yang, X.; Zhang, X.; Jia, W.; Huang, C. Enhanced cellular infiltration of tissue-engineered scaffolds fabricated by PLLA nanogrooved microfibers. Nano Res. 2022, 16, 1614–1625.
- Sheikhi, F.; Khorram, M.; Hashemi, S.-S.; Mohammadi, A.; Peyrovedin, H. Preparation, Characterization, and Surface Modification of Polycaprolactone-Based Nanofibrous Scaffold by Grafting with Collagen for Skin Tissue Engineering. Regen. Eng. Transl. Med. 2022, 8, 545–562.
- Chi, M.; Li, N.; Cui, J.; Karlin, S.; Rohr, N.; Sharma, N.; Thieringer, F.M. Biomimetic, mussel-inspired surface modification of 3D-printed biodegradable polylactic acid scaffolds with nano-hydroxyapatite for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 989729.
- Mahendiran, B.; Muthusamy, S.; Janani, G.; Mandal, B.B.; Rajendran, S.; Krishnakumar, G.S. Surface Modification of Decellularized Natural Cellulose Scaffolds with Organosilanes for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2022, 8, 2000–2015.
- Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488.
- Wang, T.; Feng, Z.-Q.; Leach, M.K.; Wu, J.; Jiang, Q. Nanoporous fibers of type-I collagen coated poly(l-lactic acid) for enhancing primary hepatocyte growth and function. J. Mater. Chem. B 2012, 1, 339–346.
- Zamani, F.; Tehran, M.A.; Latifi, M.; Shokrgozar, M.A. The influence of surface nanoroughness of electrospun PLGA nanofibrous scaffold on nerve cell adhesion and proliferation. J. Mater. Sci. Mater. Med. 2013, 24, 1551–1560.
- Meng, J.; Boschetto, F.; Yagi, S.; Marin, E.; Adachi, T.; Chen, X.; Pezzotti, G.; Sakurai, S.; Sasaki, S.; Aoki, T.; et al. Enhancing the bioactivity of melt electrowritten PLLA scaffold by convenient, green, and effective hydrophilic surface modification. Biomater. Adv. 2022, 135, 112686.
- Wałdoch, A.; Sabiniewicz, R.; Meyer-Szary, J. Interventional treatment using a 3D model of a right pulmonary artery to left atrial fistula in an infant. Adv. Interv. Cardiol. 2022, 18, 170–172.
- Chen, W.; Ma, L.; Shao, J.; Bi, C.; Xie, Y.; Zhao, S. Morphological specificity analysis of an image-based 3D model of airway filling in a difficult airway. BMC Anesthesiol. 2022, 22, 336.
- Hameed, M.; Prather, R.; Divo, E.; Kassab, A.; Nykanen, D.; Farias, M.; DeCampli, W.M. Computational fluid dynamics investigation of the novel hybrid comprehensive stage II operation. JTCVS Open 2021, 7, 308–323.
- Rossi, T.; Querzoli, G.; Badas, M.G.; Angius, F.; Telani, S.; Ripandelli, G. Computational Fluid Dynamics of Intraocular Silicone Oil Tamponade. Transl. Vis. Sci. Technol. 2021, 10, 22.
- Schollenberger, J.; Osborne, N.H.; Hernandez-Garcia, L.; Figueroa, C.A. A Combined Computational Fluid Dynamics and Arterial Spin Labeling MRI Modeling Strategy to Quantify Patient-Specific Cerebral Hemodynamics in Cerebrovascular Occlusive Disease. Front. Bioeng. Biotechnol. 2021, 9, 722445.
- Eltes, P.E.; Bartos, M.; Hajnal, B.; Pokorni, A.J.; Kiss, L.; Lacroix, D.; Varga, P.P.; Lazary, A. Development of a Computer-Aided Design and Finite Element Analysis Combined Method for Affordable Spine Surgical Navigation with 3D-Printed Customized Template. Front. Surg. 2021, 7, 583386.
- Feng, B.; Zhang, M.; Qin, C.; Zhai, D.; Wang, Y.; Zhou, Y.; Chang, J.; Zhu, Y.; Wu, C. 3D printing of conch-like scaffolds for guiding cell migration and directional bone growth. Bioact. Mater. 2022, 22, 127–140.
This entry is offline, you can click here to edit this entry!
