Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Ferroptosis is a new form of iron-dependent programmed cell death discovered, which is caused by the accumulation of lipid peroxidation (LPO) and reactive oxygen species (ROS). Studies have shown that cellular ferroptosis is closely related to tumor progression, and the induction of ferroptosis is a new means to inhibit tumor growth.
- Fe3O4 nanoparticles
- ferroptosis
1. Introduction
Global Statistics of 2020 [1] showed that there were an estimated 19.3 million new cancer cases and nearly 10 million cancer deaths, of which female breast cancer surpassed lung cancer as the most commonly diagnosed cancer with an estimated 2.3 million new cases (11.7%), followed closely by lung cancer (11.4%), colorectal cancer (10.0%), prostate cancer (7.3%), and stomach cancer (5.6%). Lung cancer remained the leading cause of cancer deaths with an estimated 1.8 million deaths (18%), followed by colorectal cancer (9.4%), liver cancer (8.3%), stomach cancer (7.7%), and female breast cancer (6.9%). The proliferation and apoptosis of corresponding tumor cells are closely related to tumor formation and progression. Thus, in the past few decades in oncology, scientific researchers have been devoted to the development of various apoptosis-related drugs to reverse the fate of non-senescent and over-proliferating tumor cells [2][3][4].
However, the therapeutic outcome is far from satisfactory due to the inherent or acquired apoptosis resistance of tumor cells [5]. Lung cancer, with the highest age-standardized incidence rate (ASIR) and age-standardized mortality rate (ASMR) worldwide, and with 2.1 million new cases and 1.8 million deaths every year, has a very high mortality rate [6][7][8][9]. Small cell lung cancer (SCLC) is one of the most malignant types of lung cancer. It has a short multiplication time, patients are prone to rapid drug resistance during treatment, and the disease deteriorates rapidly after recurrence. Currently, there is no effective second-line single-agent chemotherapy regimen other than topotecan [10]. In addition, the incidence and mortality of hepatocellular carcinoma (HCC) has rapidly increased worldwide [11]. HCC is an aggressive and highly malignant liver tumor with poor survival rates, and most patients are clinically diagnosed at an advanced stage [12]. The pathogenesis of the tumor has been of great interest to researchers; however, due to its diversity and complexity, it still has a long way to go despite the large amount of human and material resources invested every year [13][14][15][16][17]. In recent years, a new form of cell death different from the apoptotic mechanism, namely ferroptosis, has attracted attention.
Ferroptosis, first proposed in 2012 by Scott J. Dixon et al., is a unique form of iron-dependent non-apoptotic cell death triggered by the oncogenic RAS-selective lethal small molecule Erastin [18]. Early studies found that cysteine is required for the growth and death inhibition of cells, and the absence of cystine in the culture medium resulted in the death of human fibroblasts due to glutathione (GSH) depletion. Extracellular and intracellular cysteine are required to maintain GSH biosynthesis and inhibit mammalian cell death, which could be prevented and treated with the lipophilic antioxidant (α-tocopherol) and iron chelators (deferoxamine) [19]. In the following years, with the further study of the mechanism of ferroptosis, it has gradually become a promising cancer treatment.
Compared with other metal nanomaterials [20][21][22][23], Fe3O4-NPs are one of the few FDA-approved nanomaterials for clinical use, which has simple preparation [24][25][26], safe and stable chemical properties, and good biocompatibility [27][28][29]. Moreover, it has been widely used in pathogen detection [30][31][32][33][34], tumor diagnosis and treatment [35][36][37], gene mutation analysis [38][39][40][41][42], targeted drug delivery [43][44][45], and nuclear magnetic resonance imaging (MRI) [46][47][48]. In addition, recent studies have shown that Fe3O4-NPs, due to their rich Fe2+ and Fe3+ contents, can affect the iron metabolism of tumor cells, increase local ROS levels in tumor tissues, participate in or even induce the occurrence of ferroptosis in tumor cells, and then be able to inhibit or kill tumor cells.
2. Ferroptosis
Ferroptosis, a new form of cell death discovered in recent years, is an iron-dependent non-apoptotic form of cell death characterized by the accumulation of intracellular ROS [18]. This modality disrupts the redox homeostasis of cells and has a great potential to kill cancer cells [49][50]. Studies on the mechanisms of ferroptosis have focused on lipid peroxidation (LPO) and iron loading. Under normal circumstances, ROS is mainly produced by normal metabolism in mitochondria and is essential for cell signaling and tissue homeostasis [51]. Polyunsaturated fatty acid (PUFA) peroxidation induced by ROS is the main cause of ferroptosis. Furthermore, ferroptosis is regulated by a complex interaction between cellular redox homeostasis, lipid metabolism, and iron metabolism [52]. In terms of cell morphology, cells undergoing ferroptosis show some specific changes, such as the appearance of smaller mitochondria than normal, wrinkled mitochondrial membranes, reduced or absent mitochondrial cristae, and fragmented extracellular membrane [53]. The biochemical features of ferroptosis are cystine deficiency, GSH depletion, and glutathione peroxidase 4 (GPX4) inactivation [54]. However, the underlying mechanisms have not been fully elucidated.
The main cause of ferroptosis is the change of cell metabolism, which leads to the accumulation of LPO and ROS in the cells. According to existing research reports, the changes in cell metabolic pathways caused by ferroptosis mainly include the following aspects (Figure 1):
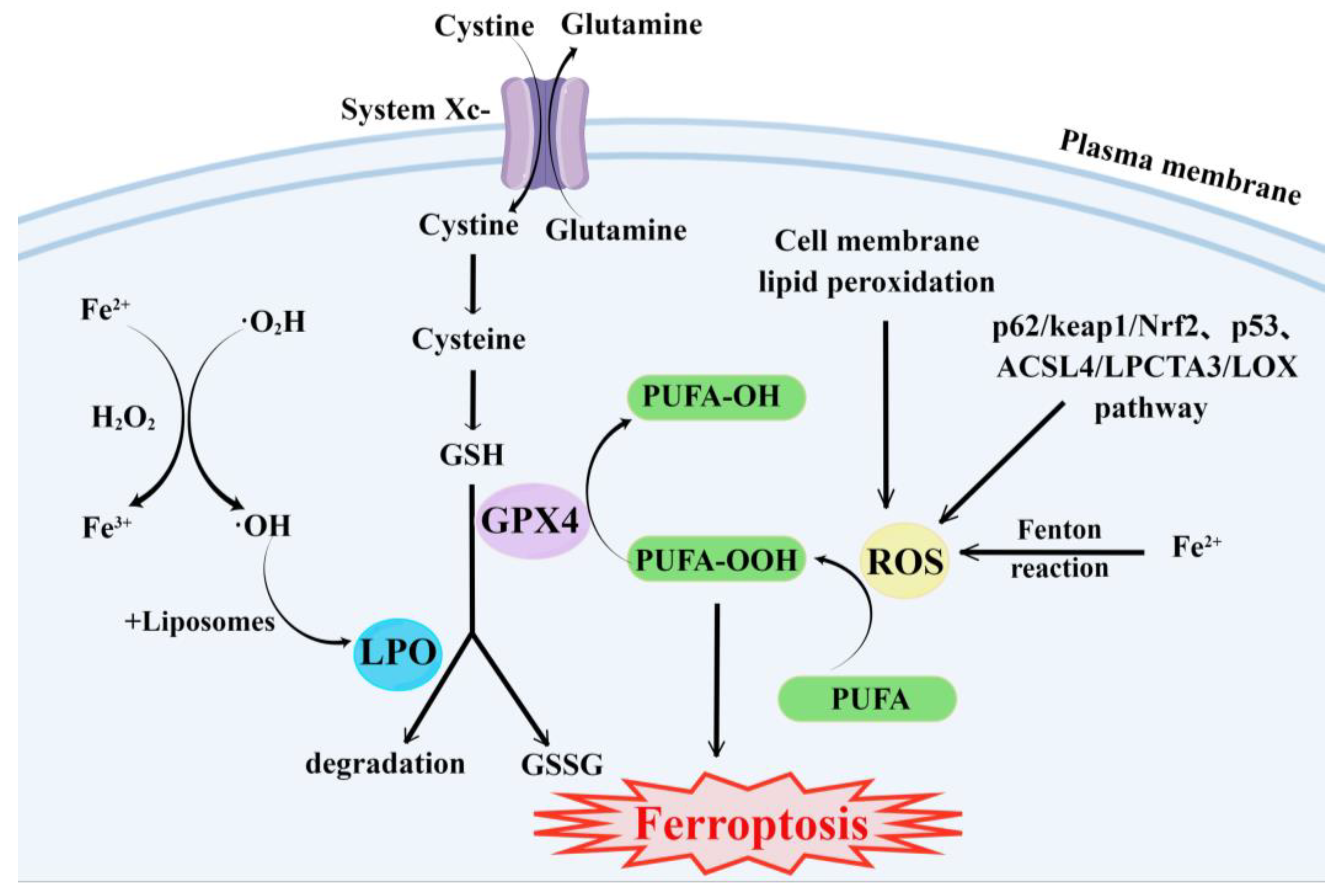
Figure 1. Major metabolic pathways of ferroptosis. By Figdraw.
- (1)
-
GSH synthesis pathway is inhibited and LPO accumulates, thus inducing ferroptosis in cells [55].
- (2)
-
Iron metabolism is altered. Iron is a redox-active metal involved in ROS formation and LPO diffusion, and a rising iron level could increase cellular susceptibility to iron-dependent cell death [56]. The accepted explanation today is that Fe2+ can transfer electrons to intracellular oxygen, and then react with intracellular lipids to form LPO, which further induces ferroptosis [57][58]. Iron metabolism genes and iron metabolism regulation genes play a key role in intracellular system iron homeostasis. For example, the gene of Iron Responsive Element Binding Protein 2 (IREB2) is a key player in the Erastin-induced ferroptosis of HT-1080 fibrosarcoma cells and Calu-1 lung cancer cells [59]. Thus, intracellular iron overload is critical for ferroptosis [60].
- (3)
-
ROS metabolic pathway effects. This pathway also plays an important role in ferroptosis. Cytosolic cystine/glutamate transport receptor (System Xc-) and voltage-dependent anion channels (VDACs) [18] in the outer mitochondrial membrane, GPX4 and ferroptosis suppressor protein 1 (FSP1) ferroptosis-related proteins [61][62], and p62/keap1/Nrf2 [63], p53-related pathway [64][65], and ACSL4/LPCTA3/LOX [66] ferroptosis-related pathways play their roles in regulating ferroptosis by affecting ROS metabolism pathways [67].
This entry is adapted from the peer-reviewed paper 10.3390/molecules28114562
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849.
- Li, F.; Wang, Z.; Huang, Y.; Xu, H.; He, L.; Deng, Y.; Zeng, X.; He, N. Delivery of PUMA Apoptosis Gene Using Polyethyleneimine-SMCC-TAT/DNA Nanoparticles: Biophysical Characterization and In Vitro Transfection Into Malignant Melanoma Cells. J. Biomed. Nanotechnol. 2015, 11, 1776–1782.
- Fu, J.; Dang, Z.; Deng, Y.; Lu, G. Regulation of c-Myc and Bcl-2 induced apoptosis of human bronchial epithelial cells by zinc oxide nanoparticles. J. Biomed. Nanotechnol. 2012, 8, 669–675.
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726.
- Li, W.; Liu, Y.; Li, Z.; Shi, Y.; Deng, J.; Bai, J.; Ma, L.; Zeng, X.; Feng, S.; Ren, J.; et al. Unravelling the Role of LncRNA WT1-AS/miR-206/NAMPT Axis as Prognostic Biomarkers in Lung Adenocarcinoma. Biomolecules 2021, 11, 203.
- Li, W.; Jia, M.; Deng, J.; Wang, J.; Lin, Q.; Tang, J.; Zeng, X.; Cai, F.; Ma, L.; Su, W.; et al. Down-regulation of microRNA-200b is a potential prognostic marker of lung cancer in southern-central Chinese population. Saudi J. Biol. Sci. 2019, 26, 173–177.
- Li, W.; Jia, M.; Wang, J.; Lu, J.; Deng, J.; Tang, J.; Liu, C. Association of MMP9-1562C/T and MMP13-77A/G Polymorphisms with Non-Small Cell Lung Cancer in Southern Chinese Population. Biomolecules 2019, 9, 107.
- Yang, S.; Guo, H.; Wei, B.; Zhu, S.; Cai, Y.; Jiang, P.; Tang, J. Association of miR-502-binding site single nucleotide polymorphism in the 3′-untranslated region of SET8 and TP53 codon 72 polymorphism with non-small cell lung cancer in Chinese population. Acta Biochim. Biophys. Sin. 2014, 46, 149–154.
- Xing, H.; Zhang, J.; Ge, F.J.; Yu, X.H.; Bian, H.M.; Zhang, F.L.; Fang, J. Analysis of the Efficacy of Irinotecan in the Second-line Treatment of Refractory and Relapsed Small Cell Lung Cancer. Chin. J. Lung Cancer 2021, 24, 167–172.
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261.
- Eggert, T.; Greten, T.F. Current Standard and Future Perspectives on Non-Surgical Therapy for Hepatocellular Carcinoma. Digestion 2017, 96, 1–4.
- Chen, J.; Zhang, S.; Wang, Y.; Xie, R.; Liu, L.; Deng, Y. In Vivo Self-Assembly Based Cancer Therapy Strategy. J. Biomed. Nanotechnol. 2020, 16, 997–1017.
- Liu, M.; Yu, X.C.; Chen, Z.; Yang, T.; Yang, D.D.; Liu, Q.Q.; Du, K.K.; Li, B.; Wang, Z.F.; Li, S.; et al. Aptamer selection and applications for breast cancer diagnostics and therapy. J. Nanobiotechnol. 2017, 15, 81.
- Liu, M.; Xi, L.; Tan, T.; Jin, L.; Wang, Z.F.; He, N.Y. A novel aptamer-based histochemistry assay for specific diagnosis of clinical breast cancer tissues. Chin. Chem. Lett. 2021, 32, 1726–1730.
- Xie, H.; Di, K.L.; Huang, R.R.; Khan, A.; Xia, Y.Y.; Xu, H.P.; Liu, C.; Tan, T.T.; Tian, X.Y.; Shen, H.; et al. Extracellular vesicles based electrochemical biosensors for detection of cancer cells: A review. Chin. Chem. Lett. 2020, 31, 1737–1745.
- Li, Z.Y.; Wang, J.H.; Yang, H.W.; Chen, S.Q.; Ma, G.J.; Zhang, X.M.; Zhu, M.; Yu, J.; Singh, R.; Zhang, Y.Y.; et al. Ultrasensitive Detection of Gastric Cancer Plasma MicroRNAs via Magnetic Beads-Based Chemiluminescent Assay. J. Biomed. Nanotechnol. 2017, 13, 1272–1280.
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072.
- Bannai, S.; Tsukeda, H.; Okumura, H. Effect of antioxidants on cultured human diploid fibroblasts exposed to cystine-free medium. Biochem. Biophys. Res. Commun. 1977, 74, 1582–1588.
- Magesa, F.; Wu, Y.Y.; Dong, S.; Tian, Y.L.; Li, G.L.; Vianney, J.M.; Buza, J.; Liu, J.; He, Q.G. Electrochemical Sensing Fabricated with Ta2O5 Nanoparticle-Electrochemically Reduced Graphene Oxide Nanocomposite for the Detection of Oxytetracycline. Biomolecules 2020, 10, 110.
- Yang, G.J.; Huang, H.; Xiao, Z.Q.; Zhang, C.X.; Guo, W.F.; Ma, T.T.; Ma, L.; Chen, Z.; Deng, Y. A Novel Strategy for Liquid Exfoliation of Ultrathin Black Phosphorus Nanosheets. J. Biomed. Nanotechnol. 2020, 16, 548–552.
- He, Q.G.; Tian, Y.L.; Wu, Y.Y.; Liu, J.; Li, G.L.; Deng, P.H.; Chen, D.C. Electrochemical Sensor for Rapid and Sensitive Detection of Tryptophan by a Cu2O Nanoparticles-Coated Reduced Graphene Oxide Nanocomposite. Biomolecules 2019, 9, 176.
- He, Q.G.; Liu, J.; Liu, X.P.; Li, G.L.; Deng, P.H.; Liang, J. Manganese dioxide Nanorods/electrochemically reduced graphene oxide nanocomposites modified electrodes for cost-effective and ultrasensitive detection of Amaranth. Colloids Surf. B-Biointerfaces 2018, 172, 565–572.
- Liu, J.; Dong, S.; He, Q.G.; Yang, S.C.; Xie, M.; Deng, P.H.; Xia, Y.H.; Li, G.L. Facile Preparation of Fe3O4/C Nanocomposite and Its Application for Cost-Effective and Sensitive Detection of Tryptophan. Biomolecules 2019, 9, 245.
- Jiang, H.R.; Zeng, X.; Xi, Z.J.; Liu, M.; Li, C.Y.; Li, Z.Y.; Jin, L.; Wang, Z.F.; Deng, Y.; He, N.Y. Improvement on Controllable Fabrication of Streptavidin-Modified Three-Layer Core-Shell Fe3O4@SiO2@Au Magnetic Nanocomposites with Low Fluorescence Background. J. Biomed. Nanotechnol. 2013, 9, 674–684.
- Ma, C.; Li, C.Y.; He, N.Y.; Wang, F.; Ma, N.N.; Zhang, L.M.; Lu, Z.X.; Ali, Z.; Xi, Z.J.; Li, X.L.; et al. Preparation and Characterization of Monodisperse Core-Shell Fe3O4@SiO2 Microspheres and Its Application for Magnetic Separation of Nucleic Acids from E. coli BL21. J. Biomed. Nanotechnol. 2012, 8, 1000–1005.
- Fan, L.; Liu, H.; Gao, C.X.; Zhu, P.Z. Facile synthesis and characterization of magnetic hydroxyapatite/Fe3O4 microspheres. Mater. Lett. 2022, 313, 131648.
- Liu, X.L.; Zhang, H.; Chang, L.; Yu, B.Z.; Liu, Q.Y.; Wu, J.P.; Miao, Y.Q.; Ma, P.; Fan, D.D.; Fan, H.M. Human-like collagen protein-coated magnetic nanoparticles with high magnetic hyperthermia performance and improved biocompatibility. Nanoscale Res. Lett. 2015, 10, 28.
- Zhao, Y.; Fan, T.T.; Chen, J.D.; Su, J.C.; Zhi, X.; Pan, P.P.; Zou, L.; Zhang, Q.Q. Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloids Surf. B-Biointerfaces 2019, 174, 70–79.
- He, L.; Yang, H.W.; Xiao, P.F.; Singh, R.; He, N.Y.; Liu, B.; Li, Z.Y. Highly Selective, Sensitive and Rapid Detection of Escherichia coli O157: H7 Using Duplex PCR and Magnetic Nanoparticle-Based Chemiluminescence Assay. J. Biomed. Nanotechnol. 2017, 13, 1243–1252.
- Ling, Y.Z.; Zhu, Y.F.; Fan, H.H.; Zha, H.L.; Yang, M.; Wu, L.; Chen, H.; Li, W.; Wu, Y.P.; Chen, H.B. Rapid Method for Detection of Staphylococcus aureus in Feces. J. Biomed. Nanotechnol. 2019, 15, 1290–1298.
- Liu, H.N.; Dong, H.M.; Chen, Z.; Lin, L.; Chen, H.; Li, S.; Deng, Y. Magnetic Nanoparticles Enhanced Microarray Detection of Multiple Foodborne Pathogens. J. Biomed. Nanotechnol. 2017, 13, 1333–1343.
- Li, S.; Liu, H.N.; Deng, Y.; Lin, L.; He, N.Y. Development of a Magnetic Nanoparticles Microarray for Simultaneous and Simple Detection of Foodborne Pathogens. J. Biomed. Nanotechnol. 2013, 9, 1254–1260.
- Mou, X.B.; Chen, Z.; Li, T.T.; Liu, M.; Liu, Y.; Ali, Z.; Li, S.; Zhu, Y.B.; Li, Z.Y.; Deng, Y. A Highly Sensitive Strategy for Low-Abundance Hepatitis B Virus Detection via One-Step Nested Polymerase Chain Reaction, Chemiluminescence Technology and Magnetic Separation. J. Biomed. Nanotechnol. 2019, 15, 1832–1838.
- Hao, H.Q.; Ma, Q.M.; He, F.; Yao, P. Doxorubicin and Fe3O4 loaded albumin nanoparticles with folic acid modified dextran surface for tumor diagnosis and therapy. J. Mater. Chem. B 2014, 2, 7978–7987.
- Wang, Q.; Zhao, X.M.; Yan, H.; Kang, F.Y.; Li, Z.F.; Qiao, Y.Y.; Li, D. A cross-talk EGFR/VEGFR-targeted bispecific nanoprobe for magnetic resonance/near-infrared fluorescence imaging of colorectal cancer. MRS Commun. 2018, 8, 1008–1017.
- Wang, M.L.; Yang, Q.M.; Li, M.; Zou, H.M.; Wang, Z.G.; Ran, H.T.; Zheng, Y.Y.; Jian, J.; Zhou, Y.; Luo, Y.D.; et al. Multifunctional Nanoparticles for Multimodal Imaging-Guided Low-Intensity Focused Ultrasound/Immunosynergistic Retinoblastoma Therapy. ACS Appl. Mater. Interfaces 2020, 12, 5642–5657.
- Tang, Y.J.; Ali, Z.; Dai, J.G.; Liu, X.L.; Wu, Y.Q.; Chen, Z.; He, N.Y.; Li, S.; Wang, L.J. Single-Nucleotide Polymorphism Genotyping of exoS in Pseudomonas aeruginosa Using Dual-Color Fluorescence Hybridization and Magnetic Separation. J. Biomed. Nanotechnol. 2018, 14, 206–214.
- Mou, X.B.; Sheng, D.N.; Chen, Z.; Liu, M.; Liu, Y.; Deng, Y.; Xu, K.; Hou, R.X.; Zhao, J.Y.; Zhu, Y.B.; et al. In-Situ Mutation Detection by Magnetic Beads-Probe Based on Single Base Extension and Its Application in Genotyping of Hepatitis B Virus Pre-C Region 1896nt Locus Single Nucleotide Polymorphisms. J. Biomed. Nanotechnol. 2019, 15, 2393–2400.
- Liu, B.; Jia, Y.Y.; Ma, M.; Li, Z.Y.; Liu, H.N.; Li, S.; Deng, Y.; Zhang, L.M.; Lu, Z.X.; Wang, W.; et al. High Throughput SNP Detection System Based on Magnetic Nanoparticles Separation. J. Biomed. Nanotechnol. 2013, 9, 247–256.
- Li, S.; Liu, H.N.; Jia, Y.Y.; Mou, X.B.; Deng, Y.; Lin, L.; Liu, B.; He, N.Y. An Automatic High-Throughput Single Nucleotide Polymorphism Genotyping Approach Based on Universal Tagged Arrays and Magnetic Nanoparticles. J. Biomed. Nanotechnol. 2013, 9, 689–698.
- Tang, C.L.; He, Z.Y.; Liu, H.M.; Xu, Y.Y.; Huang, H.; Yang, G.J.; Xiao, Z.Q.; Li, S.; Liu, H.N.; Deng, Y.; et al. Application of magnetic nanoparticles in nucleic acid detection. J. Nanobiotechnol. 2020, 18, 62.
- Wang, Y.Z.; Shi, Z.; Sun, Y.; Wu, X.Y.; Li, S.; Dong, S.B. Preparation of amphiphilic magnetic polyvinyl alcohol targeted drug carrier and drug delivery research. Des. Monomers Polym. 2020, 23, 197–206.
- Shen, L.Z.; Li, B.; Qiao, Y.S. Fe3O4 Nanoparticles in Targeted Drug/Gene Delivery Systems. Materials 2018, 11, 324.
- Yew, Y.P.; Shameli, K.; Miyake, M.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Naiki, T.; Lee, K.X. Green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug delivery system: A review. Arab. J. Chem. 2020, 13, 2287–2308.
- Hu, Y.; Mignani, S.; Majoral, J.P.; Shen, M.W.; Shi, X.Y. Construction of iron oxide nanoparticle-based hybrid platforms for tumor imaging and therapy. Chem. Soc. Rev. 2018, 47, 1874–1900.
- Wang, K.; Xu, X.G.; Ma, Y.L.; Sheng, C.R.; Li, L.N.; Lu, L.Y.; Wang, J.; Wang, Y.N.; Jiang, Y. Fe3O4@Angelica sinensis polysaccharide nanoparticles as an ultralow-toxicity contrast agent for magnetic resonance imaging. Rare Met. 2021, 40, 2486–2493.
- Li, J.J.; Wang, J.H.; Li, J.Y.; Yang, X.; Wan, J.L.; Zheng, C.S.; Du, Q.; Zhou, G.F.; Yang, X.L. Fabrication of Fe3O4@PVA microspheres by one-step electrospray for magnetic resonance imaging during transcatheter arterial embolization. Acta Biomater. 2021, 131, 532–543.
- Wu, C.H.; Zhao, W.W.; Yu, J.; Li, S.J.; Lin, L.G.; Chen, X.P. Induction of ferroptosis and mitochondrial dysfunction by oxidative stress in PC12 cells. Sci. Rep. 2018, 8, 574.
- Chen, Y.; Liu, Y.; Lan, T.; Qin, W.; Zhu, Y.T.; Qin, K.; Gao, J.J.; Wang, H.B.; Hou, X.M.; Chen, N.; et al. Quantitative Profiling of Protein Carbonylations in Ferroptosis by an Aniline-Derived Probe. J. Am. Chem. Soc. 2018, 140, 4712–4720.
- Latunde-Dada, G.O. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 1893–1900.
- Zhu, L.Y.; Meng, D.N.; Wang, X.; Chen, X.R. Ferroptosis-Driven Nanotherapeutics to Reverse Drug Resistance in Tumor Microenvironment. ACS Appl. Bio Mater. 2022, 5, 2481–2506.
- Dixon, S.J. Ferroptosis: Bug or feature? Immunol. Rev. 2017, 277, 150–157.
- Jiang, M.Y.; Hu, R.L.; Yu, R.X.; Tang, Y.W.; Li, J.R. A narrative review of mechanisms of ferroptosis in cancer: New challenges and opportunities. Ann. Transl. Med. 2021, 9, 20.
- Wu, J.M.; Ma, L.F.; Wang, J.Y.; Qiao, Y.X. Mechanism of Ferroptosis and Its Research Progress in Lung Cancer. Chin. J. Lung Cancer 2020, 23, 811–817.
- Gao, M.H.; Monian, P.; Pan, Q.H.; Zhang, W.; Xiang, J.; Jiang, X.J. Ferroptosis is an autophagic cell death process. Cell Res. 2016, 26, 1021–1032.
- He, Y.J.; Liu, X.Y.; Xing, L.; Wan, X.; Chang, X.; Jiang, H.L. Fenton reaction-independent ferroptosis therapy via glutathione and iron redox couple sequentially triggered lipid peroxide generator. Biomaterials 2020, 241, 119911.
- Liu, T.; Liu, W.L.; Zhang, M.K.; Yu, W.Y.; Gao, F.; Li, C.X.; Wang, S.B.; Feng, J.; Zhang, X.Z. Ferrous-Supply-Regeneration Nanoengineering for Cancer-Cell-Specific Ferroptosis in Combination with Imaging-Guided Photodynamic Therapy. ACS Nano 2018, 12, 12181–12192.
- Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Regulators of Iron Homeostasis: New Players in Metabolism, Cell Death, and Disease. Trends Biochem. Sci. 2016, 41, 274–286.
- Angeli, J.P.F.; Krysko, D.V.; Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 2019, 19, 405–414.
- Imai, H.; Matsuoka, M.; Kumagai, T.; Sakamoto, T.; Koumura, T. Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis. In Apoptotic and Non-Apoptotic Cell Death; Nagata, S., Nakano, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 143–170.
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Grocin, A.G.; da Silva, T.N.X.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698.
- Bartolini, D.; Dallaglio, K.; Torquato, P.; Piroddi, M.; Galli, F. Nrf2-p62 autophagy pathway and its response to oxidative stress in hepatocellular carcinoma. Transl. Res. 2018, 193, 54–71.
- Kang, R.; Kroemer, G.; Tang, D.L. The tumor suppressor protein p53 and the ferroptosis network. Free Radic. Biol. Med. 2019, 133, 162–168.
- Chu, B.; Kon, N.; Chen, D.L.; Li, T.Y.; Liu, T.; Jiang, L.; Song, S.J.; Tavana, O.; Gu, W. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat. Cell Biol. 2019, 21, 579–591.
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; IngoId, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98.
- Shen, Z.; Song, J.; Yung, B.C.; Zhou, Z.; Wu, A.; Chen, X. Emerging Strategies of Cancer Therapy Based on Ferroptosis. Adv. Mater. 2018, 30, e1704007.
This entry is offline, you can click here to edit this entry!
