The actinium-225 (225Ac) radioisotope exhibits highly attractive nuclear properties for application in radionuclide therapy. However, the 225Ac radionuclide presents multiple daughter nuclides in its decay chain, which can escape the targeted site, circulate in plasma, and cause toxicity in areas such as kidneys and renal tissues. Several ameliorative strategies have been devised to circumvent this issue, including nano-delivery. Alpha-emitting radionuclides and nanotechnology applications in nuclear medicine have culminated in major advancements that offer promising therapeutic possibilities for treating several cancers. Accordingly, the importance of nanomaterials in retaining the 225Ac daughters from recoiling into unintended organs has been established. Researchers described the advancements of targeted radionuclide therapy (TRT) as an alternative anticancer treatment. It discusses the recent developments in the preclinical and clinical investigations on 225Ac as a prospective anticancer agent. Moreover, the rationale for using nanomaterials in improving the therapeutic efficacy of α-particles in targeted alpha therapy (TAT) with an emphasis on 225Ac is discussed. Quality control measures in the preparation of 225Ac-conjugates are also highlighted.
- actinium-225
- recoiling of daughters
- targeted radionuclide therapy
- radiopharmaceuticals
- nanotechnology
1. Introduction
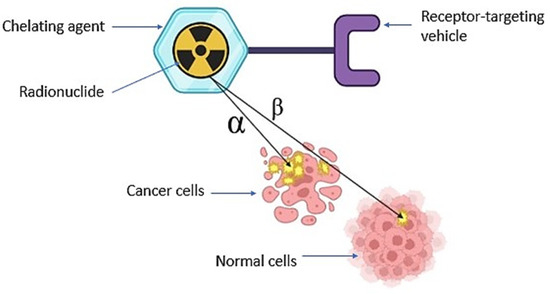
2. Targeted Radionuclide Therapy in Cancer Management
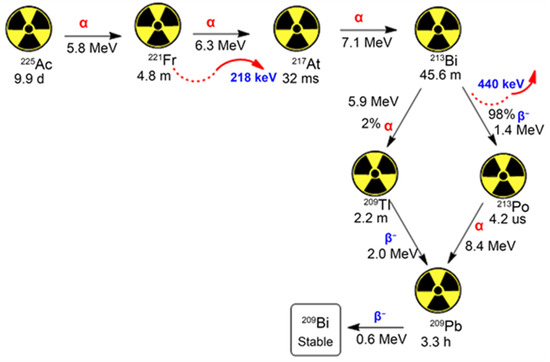
3. Recent Investigations on Actinium-225 as a Prospective Therapeutic Agent
3.1. Pre-Clinical Studies
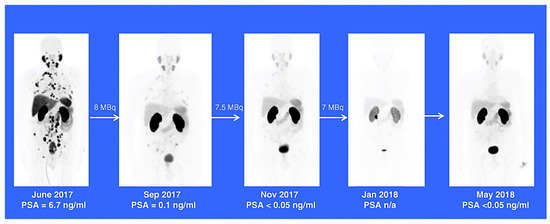
3.2. Clinical Studies
4. Nanodelivery of Radionuclides
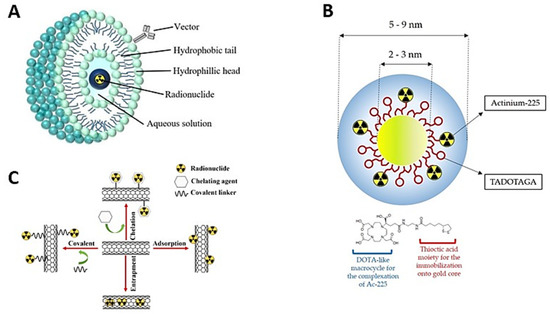
Nanodelivery of Actinium-225
5. Quality Control and Preparation of Actinium-255 Conjugates
6. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15061719
References
- Ferrier, M.G.; Batista, E.R.; Berg, J.M.; Birnbaum, E.R.; Cross, J.N.; Engle, J.W.; La Pierre, H.S.; Kozimor, S.A.; Pacheco, J.S.L.; Stein, B.W.; et al. Spectroscopic and computational investigation of actinium coordination chemistry. Nat. Commun. 2016, 7, 12312.
- Yamana, H.; Mitsugashira, T.; Shiokawa, Y.; Sato, A.; Suzuki, S. Possibility of the existence of divalent actinium in aqueous solution. J. Radioanal. Nucl. Chem. 1983, 76, 19–26.
- Miederer, M.; Scheinberg, D.A.; McDevitt, M.R. Realizing the potential of the Actinium-225 radionuclide generator in targeted alpha particle therapy applications. Adv. Drug Deliv. Rev. 2008, 60, 1371–1382.
- Morgenstern, A.; Apostolidis, C.; Bruchertseifer, F. Supply and Clinical Application of Actinium-225 and Bismuth-213. Semin. Nucl. Med. 2020, 50, 119–123.
- Sen, I.B.; Thakral, P.; Simecek, J.; Marx, S.; Kumari, J.; Pant, V. In-house preparation and quality control of Ac-225 prostate-specific membrane antigen-617 for the targeted alpha therapy of castration-resistant prostate carcinoma. Indian J. Nucl. Med. 2021, 36, 114–119.
- Fitzsimmons, J.; Atcher, R. Synthesis and evaluation of a water-soluble polymer to reduce Ac-225 daughter migration. J. Label. Compd. Radiopharm. 2007, 50, 147–153.
- McDevitt, M.R.; A Scheinberg, D. Ac-225 and her daughters: The many faces of Shiva. Cell Death Differ. 2002, 9, 593–594.
- Muslimov, A.R.; Antuganov, D.; Tarakanchikova, Y.V.; Karpov, T.E.; Zhukov, M.V.; Zyuzin, M.V.; Timin, A.S. An investigation of calcium carbonate core-shell particles for incorporation of 225Ac and sequester of daughter radionuclides: In vitro and in vivo studies. J. Control. Release 2021, 330, 726–737.
- Ngema, L.M.; Adeyemi, S.A.; Marimuthu, T.; Choonara, Y.E. A review on engineered magnetic nanoparticles in Non-Small-Cell lung carcinoma targeted therapy. Int. J. Pharm. 2021, 606, 120870.
- Kleynhans, J.; Sathekge, M.; Ebenhan, T. Obstacles and Recommendations for Clinical Translation of Nanoparticle System-Based Targeted Alpha-Particle Therapy. Materials 2021, 14, 4784.
- Kim, Y.-S.; Brechbiel, M.W. An overview of targeted alpha therapy. Tumor Biol. 2011, 33, 573–590.
- Phua, V.J.X.; Yang, C.-T.; Xia, B.; Yan, S.X.; Liu, J.; Aw, S.E.; He, T.; Ng, D.C.E. Nanomaterial Probes for Nuclear Imaging. Nanomaterials 2022, 12, 582.
- Peltek, O.O.; Muslimov, A.R.; Zyuzin, M.V.; Timin, A.S. Current outlook on radionuclide delivery systems: From design consideration to translation into clinics. J. Nanobiotechnol. 2019, 17, 90.
- Alkandari, A.M.; Alsayed, Y.M.; El-Hanbaly, A.M. Radiopharmaceutical encapsulated liposomes as a novel radiotracer imaging and drug delivery protocol. Curr. Radiopharm. 2022, 16, 133–139.
- Kerr, C.P.; Grudzinski, J.J.; Nguyen, T.P.; Hernandez, R.; Weichert, J.P.; Morris, Z.S. Developments in Combining Targeted Radionuclide Therapies and Immunotherapies for Cancer Treatment. Pharmaceutics 2022, 15, 128.
- Herrmann, K.; Schwaiger, M.; Lewis, J.S.; Solomon, S.B.; McNeil, B.J.; Baumann, M.; Gambhir, S.S.; Hricak, H.; Weissleder, R. Radiotheranostics: A roadmap for future development. Lancet Oncol. 2020, 21, e146–e156.
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608.
- Thiele, N.A.; Wilson, J.J. Actinium-225 for Targeted α Therapy: Coordination Chemistry and Current Chelation Approaches. Cancer Biother. Radiopharm. 2018, 33, 336–348.
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Murphy, D.G.; et al. -PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833.
- Coldwell, D.; Sangro, B.; Salem, R.; Wasan, H.; Kennedy, A. Radioembolization in the Treatment of Unresectable Liver Tumors: Experience across a range of primary cancers. Am. J. Clin. Oncol. 2012, 35, 167–177.
- Witzig, T.E.; Gordon, L.I.; Cabanillas, F.; Czuczman, M.S.; Emmanouilides, C.; Joyce, R.; Pohlman, B.L.; Bartlett, N.L.; Wiseman, G.A.; Padre, N.; et al. Randomized Controlled Trial of Yttrium-90–Labeled Ibritumomab Tiuxetan Radioimmunotherapy Versus Rituximab Immunotherapy for Patients with Relapsed or Refractory Low-Grade, Follicular, or Transformed B-Cell Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2002, 20, 2453–2463.
- Bernier, M.-O.; Leenhardt, L.; Hoang, C.; Aurengo, A.; Mary, J.-Y.; Menegaux, F.; Enkaoua, E.; Turpin, G.; Chiras, J.; Saillant, G.; et al. Survival and Therapeutic Modalities in Patients with Bone Metastases of Differentiated Thyroid Carcinomas. J. Clin. Endocrinol. Metab. 2001, 86, 1568–1573.
- Majkowska-Pilip, A.; Gawęda, W.; Żelechowska-Matysiak, K.; Wawrowicz, K.; Bilewicz, A. Nanoparticles in targeted alpha therapy. Nanomaterials 2020, 10, 1366.
- Kennel, S.J.; Brechbiel, M.W.; Milenic, D.E.; Schlom, J.; Mirzadeh, S. Actinium-225 conjugates of MAb CC49 and humanized δCH2CC49. Cancer Biother. Radiopharm. 2002, 17, 219–231.
- Reissig, F.; Bauer, D.; Zarschler, K.; Novy, Z.; Bendova, K.; Ludik, M.-C.; Kopka, K.; Pietzsch, H.-J.; Petrik, M.; Mamat, C. Towards Targeted Alpha Therapy with Actinium-225: Chelators for Mild Condition Radiolabeling and Targeting PSMA—A Proof of Concept Study. Cancers 2021, 13, 1974.
- Beckford-Vera, D.; Li, J.; Jennings, C.; McCloskey, M.; Chin, A.; Liang, Q.; Hwang, J.; Roy, M.; Chen, M.; Kotanides, H. Abstract 609: Anti-HER3 radioimmunotherapy enhances the anti-tumor effects of CD47 blockade in solid tumors. Cancer Res. 2022, 82, 609.
- Seidl, C. Targets for therapy of bladder cancer. Semin. Nucl. Med. 2020, 50, 162–170.
- Dekempeneer, Y.; Caveliers, V.; Ooms, M.; Maertens, D.; Gysemans, M.; Lahoutte, T.; Xavier, C.; Lecocq, Q.; Maes, K.; Covens, P.; et al. Therapeutic Efficacy of 213Bi-labeled sdAbs in a Preclinical Model of Ovarian Cancer. Mol. Pharm. 2020, 17, 3553–3566.
- Sindhu, K.K.; Nehlsen, A.D.; Stock, R.G. Radium-223 for Metastatic Castrate-Resistant Prostate Cancer. Pract. Radiat. Oncol. 2022, 12, 312–316.
- Dolgin, E. Radioactive drugs emerge from the shadows to storm the market. Nat. Biotechnol. 2018, 36, 1125–1127.
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103.
- Pouget, J.-P.; Lozza, C.; Deshayes, E.; Boudousq, V.; Navarro-Teulon, I. Introduction to Radiobiology of Targeted Radionuclide Therapy. Front. Med. 2015, 2, 12.
- Cakici, D.; Kilbas, B. Synthesis and Stability Studies of 225Actinium Tin Colloid Radiopharmaceutical. Adv. Nanoparticles 2022, 11, 23–30.
- Scheinberg, D.A.; McDevitt, M.R. Actinium-225 in targeted alpha-particle therapeutic applications. Curr. Radiopharm. 2011, 4, 306–320.
- Ruigrok, E.A.M.; Tamborino, G.; de Blois, E.; Roobol, S.J.; Verkaik, N.; De Saint-Hubert, M.; Konijnenberg, M.W.; van Weerden, W.M.; de Jong, M.; Nonnekens, J. In vitro dose effect relationships of actinium-225- and lutetium-177-labeled PSMA-I&T. Eur. J. Nucl. Med. 2022, 49, 3627–3638.
- Borchardt, P.E.; Yuan, R.R.; Miederer, M.; McDevitt, M.R.; Scheinberg, D.A. Targeted actinium-225 in vivo generators for therapy of ovarian cancer. Cancer Res. 2003, 63, 5084–5090.
- Ballangrud, A.M.; Yang, W.-H.; Palm, S.; Enmon, R.; Borchardt, P.E.; Pellegrini, V.A.; McDevitt, M.R.; Scheinberg, D.A.; Sgouros, G. Alpha-particle emitting atomic generator (actinium-225)-labeled trastuzumab (herceptin) targeting of breast cancer spheroids: Efficacy versus HER2/neu expression. Clin. Cancer Res. 2004, 10, 4489–4497.
- Yang, H.; Zhang, C.; Yuan, Z.; Rodriguez-Rodriguez, C.; Robertson, A.; Radchenko, V.; Perron, R.; Gendron, D.; Causey, P.; Gao, F. Synthesis and evaluation of a macrocyclic actinium-225 chelator, quality control and in vivo evaluation of 225Ac-crown-αMSH peptide. Chem.-Eur. J. 2020, 26, 11435–11440.
- de Saint-Hubert, M.; Crabbe, M.; Struelens, L.; Koole, M. Targeted alpha therapy: A critical review of translational dosimetry research with emphasis on actinium-225. Q. J. Nucl. Med. Mol. Imaging 2020, 64, 265–277.
- Deblonde, G.J.-P.; Mattocks, J.A.; Dong, Z.; Wooddy, P.T.; Cotruvo, J.A.; Zavarin, M. Capturing an elusive but critical element: Natural protein enables actinium chemistry. Sci. Adv. 2021, 7, eabk0273.
- Reissig, F.; Zarschler, K.; Novy, Z.; Petrik, M.; Bendova, K.; Kurfurstova, D.; Bouchal, J.; Ludik, M.-C.; Brandt, F.; Kopka, K.; et al. Modulating the pharmacokinetic profile of Actinium-225-labeled macropa-derived radioconjugates by dual targeting of PSMA and albumin. Theranostics 2022, 12, 7203–7215.
- Bruchertseifer, F.; Apostolidis, C. Bismuth-213 and Actinium-225—Generator Performance and Evolving Therapeutic Applications of Two Generator-Derived Alpha-Emitting Radioisotopes. Curr. Radiopharm. 2012, 5, 221–227.
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: A pilot study. Eur. J. Nucl. Med. 2018, 46, 129–138.
- Sood, A.; Satapathy, S.; Mittal, B.R.; Das, C.K.; Singh, S.K.; Mavuduru, R.S.; Bora, G.S. Health-related quality-of-life outcomes with actinium-225-prostate-specific membrane antigen-617 therapy in patients with heavily pretreated metastatic castration-resistant prostate cancer. Indian J. Nucl. Med. 2020, 35, 299–304.
- Lawal, I.O.; Morgenstern, A.; Vorster, M.; Knoesen, O.; Mahapane, J.; Hlongwa, K.N.; Maserumule, L.C.; Ndlovu, H.; Reed, J.D.; Popoola, G.O.; et al. Hematologic toxicity profile and efficacy of Ac-PSMA-617 α-radioligand therapy of patients with extensive skeletal metastases of castration-resistant prostate cancer. Eur. J. Nucl. Med. 2022, 49, 3581–3592.
- Jurcic, J.G. Clinical Studies with Bismuth-213 and Actinium-225 for Hematologic Malignancies. Curr. Radiopharm. 2018, 11, 192–199.
- Jurcic, J.G.; Ravandi, F.; Pagel, J.M.; Park, J.H.; Smith, B.D.; Douer, D.; Levy, M.Y.; Estey, E.; Kantarjian, H.M.; Earle, D.; et al. Phase I trial of α-particle therapy with actinium-225 (225Ac)-lintuzumab (anti-CD33) and low-dose cytarabine (LDAC) in older patients with untreated acute myeloid leukemia (AML). J. Clin. Oncol. 2015, 33, 7050.
- Finn, L.E.; Levy, M.; Orozco, J.J.; Park, J.H.; Atallah, E.; Craig, M.; Perl, A.E.; Scheinberg, D.A.; Cicic, D.; Bergonio, G.R. A phase 2 study of actinium-225 (225Ac)-lintuzumab in older patients with previously untreated acute myeloid leukemia (AML) unfit for intensive chemotherapy. Blood 2017, 130, 2638.
- Rosenblat, T.L.; McDevitt, M.R.; Carrasquillo, J.A.; Pandit-Taskar, N.; Frattini, M.G.; Maslak, P.G.; Park, J.H.; Douer, D.; Cicic, D.; Larson, S.M.; et al. Treatment of Patients with Acute Myeloid Leukemia with the Targeted Alpha-Particle Nanogenerator Actinium-225-Lintuzumab. Clin. Cancer Res. 2022, 28, 2030–2037.
- Mironidou-Tzouveleki, M.; Tsartsalis, S. Nanotechnology and radiopharmaceuticals: Diagnostic and therapeutic approaches. Curr. Drug Deliv. 2010, 7, 168–174.
- Kleynhans, J.; Grobler, A.F.; Ebenhan, T.; Sathekge, M.M.; Zeevaart, J.-R. Radiopharmaceutical enhancement by drug delivery systems: A review. J. Control. Release 2018, 287, 177–193.
- Salvanou, E.-A.; Stellas, D.; Tsoukalas, C.; Mavroidi, B.; Paravatou-Petsotas, M.; Kalogeropoulos, N.; Xanthopoulos, S.; Denat, F.; Laurent, G.; Bazzi, R.; et al. A Proof-of-Concept Study on the Therapeutic Potential of Au Nanoparticles Radiolabeled with the Alpha-Emitter Actinium-225. Pharmaceutics 2020, 12, 188.
- Jaymand, M.; Taghipour, Y.D.; Rezaei, A.; Derakhshankhah, H.; Abazari, M.F.; Samadian, H.; Hamblin, M.R. Radiolabeled carbon-based nanostructures: New radiopharmaceuticals for cancer therapy? Coord. Chem. Rev. 2021, 440, 213974.
- Govaert, G.A.; Ijpma, F.F.; McNally, M.; McNally, E.; Reininga, I.H.; Glaudemans, A.W. Accuracy of diagnostic imaging modalities for peripheral post-traumatic osteomyelitis—A systematic review of the recent literature. Eur. J. Nucl. Med. 2017, 44, 1393–1407.
- Zhang, Y.; Sun, Y.; Xu, X.; Zhu, H.; Huang, L.; Zhang, X.; Qi, Y.; Shen, Y.-M. Radiosynthesis and micro-SPECT imaging of 99mTc-dendrimer poly (amido)-amine folic acid conjugate. Bioorg. Med. Chem. Lett. 2010, 20, 927–931.
- Criscione, J.M.; Dobrucki, L.W.; Zhuang, Z.W.; Papademetris, X.; Simons, M.; Sinusas, A.J.; Fahmy, T.M. Development and Application of a Multimodal Contrast Agent for SPECT/CT Hybrid Imaging. Bioconjugate Chem. 2011, 22, 1784–1792.
- Cędrowska, E.; Pruszynski, M.; Majkowska-Pilip, A.; Męczyńska-Wielgosz, S.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Functionalized TiO2 nanoparticles labelled with 225Ac for targeted alpha radionuclide therapy. J. Nanoparticle Res. 2018, 20, 83.
- Silindir-Gunay, M.; Karpuz, M.; Ozer, A.Y. Targeted Alpha Therapy and Nanocarrier Approach. Cancer Biother. Radiopharm. 2020, 35, 446–458.
- Henriksen, G.; Schoultz, B.W.; Michaelsen, T.E.; Bruland, Ø.S.; Larsen, R.H. Sterically stabilized liposomes as a carrier for α-emitting radium and actinium radionuclides. Nucl. Med. Biol. 2004, 31, 441–449.
- Sofou, S.; Kappel, B.J.; Jaggi, J.S.; McDevitt, M.R.; Scheinberg, D.A.; Sgouros, G. Enhanced Retention of the α-Particle-Emitting Daughters of Actinium-225 by Liposome Carriers. Bioconjugate Chem. 2007, 18, 2061–2067.
- Sofou, S.; Thomas, J.L.; Lin, H.-Y.; McDevitt, M.R.; Scheinberg, D.A.; Sgouros, G. Engineered liposomes for potential α-particle therapy of metastatic cancer. J. Nucl. Med. 2004, 45, 253–260.
- De Kruijff, R.M.; Raavé, R.; Kip, A.; Molkenboer-Kuenen, J.; Morgenstern, A.; Bruchertseifer, F.; Heskamp, S.; Denkova, A.G. The in vivo fate of 225Ac daughter nuclides using polymersomes as a model carrier. Sci. Rep. 2019, 9, 11671.
- de Kruijff, R.; Drost, K.; Thijssen, L.; Morgenstern, A.; Bruchertseifer, F.; Lathouwers, D.; Wolterbeek, H.; Denkova, A. Improved 225Ac daughter retention in InPO4 containing polymersomes. Appl. Radiat. Isot. 2017, 128, 183–189.
- Matson, M.L.; Villa, C.H.; Ananta, J.S.; Law, J.J.; Scheinberg, D.A.; Wilson, L.J. Encapsulation of α-particle-emitting 225Ac3+ ions within carbon nanotubes. J. Nucl. Med. 2015, 56, 897–900.
- McLaughlin, M.F.; Robertson, D.; Pevsner, P.H.; Wall, J.S.; Mirzadeh, S.; Kennel, S.J. LnPO4 Nanoparticles Doped with Ac-225 and Sequestered Daughters for Targeted Alpha Therapy. Cancer Biother. Radiopharm. 2014, 29, 34–41.
- Toro-González, M.; Dame, A.N.; Mirzadeh, S.; Rojas, J.V. Gadolinium vanadate nanocrystals as carriers of α-emitters (225Ac, 227Th) and contrast agents. J. Appl. Phys. 2019, 125, 214901.
- Lange, R.; ter Heine, R.; Decristoforo, C.; Peñuelas, I.; Elsinga, P.H.; van der Westerlaken, M.M.; Hendrikse, N.H. Untangling the web of European regulations for the preparation of unlicensed radiopharmaceuticals: A concise overview and practical guidance for a risk-based approach. Nucl. Med. Commun. 2015, 36, 414–422.
- Hooijman, E.L.; Ntihabose, C.M.; Reuvers, T.G.A.; Nonnekens, J.; Aalbersberg, E.A.; van de Merbel, J.R.J.P.; Huijmans, J.E.; Koolen, S.L.W.; Hendrikx, J.J.M.A.; de Blois, E. Radiolabeling and quality control of therapeutic radiopharmaceuticals: Optimization, clinical implementation and comparison of radio-TLC/HPLC analysis, demonstrated by Lu-PSMA. EJNMMI Radiopharm. Chem. 2022, 7, 29.
- Apostolidis, C.; Molinet, R.; Rasmussen, G.; Morgenstern, A. Production of 225Ac from 229Th for targeted α therapy. Anal. Chem. 2005, 77, 6288–6291.
- Abou, D.S.; Zerkel, P.; Robben, J.; McLaughlin, M.; Hazlehurst, T.; Morse, D.; Wadas, T.J.; Pandya, D.N.; Oyama, R.; Gaehle, G.; et al. Radiopharmaceutical Quality Control Considerations for Accelerator-Produced Actinium Therapies. Cancer Biother. Radiopharm. 2022, 37, 355–363.
- Kelly, J.M.; Amor-Coarasa, A.; Sweeney, E.; Wilson, J.J.; Causey, P.W.; Babich, J.W. A suitable time point for quantifying the radiochemical purity of 225Ac-labeled radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2021, 6, 38.
- Hooijman, E.L.; Chalashkan, Y.; Ling, S.W.; Kahyargil, F.F.; Segbers, M.; Bruchertseifer, F.; Morgenstern, A.; Seimbille, Y.; Koolen, S.L.W.; Brabander, T.; et al. Development of Ac-PSMA-I&T for Targeted Alpha Therapy According to GMP Guidelines for Treatment of mCRPC. Pharmaceutics 2021, 13, 715.
- Scott, P.J.H.; Dumond, A.R.S.; Rodnick, M.E.; Piert, M.R. Synthesis of 225Ac-PSMA-617 for preclinical use. Curr. Radiopharm. 2021, 15, 96–103.
